Abstract
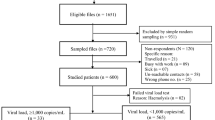
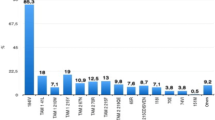
HIV-1 transmitted drug resistance (TDR) mutations may reduce the efficacy of antiretroviral therapy (ART), but pre-treatment testing to determine the virus genotype can improve the efficacy of ART. Unfortunately, issues related to cost and logistics of pre-treatment testing limit its use in resource-limited settings. We studied 596 ART-naive individuals who were newly diagnosed from 2014 to 2016 in São Paulo, Brazil, to evaluate TDR and virological outcome after 48 weeks of genotype-guided therapy. One or more TDR (based on the WHO surveillance list) was observed in 10.9% (CI 95%, 8.6–13.6) of the sequences, the most common of which was the K103 N mutation, which confers resistance to first-generation drugs of the non-nucleoside reverse transcriptase inhibitor (NNRTI) antiretroviral drug class. Dual-class (1%, 6/596) and triple-class (0.34%, 2/596) resistance were uncommon. After 48 weeks of treatment with ART, infection was suppressed to below 200 copies/mL in most patients (95%), with full suppression (RNA target not detected) in 65%. The following characteristics at patient enrollment were independently associated with a lack of full suppression: CD4 T cell counts below 500 cells/µL, viremia above 100,000 copies/mL, older age, and TDR to NNRTI. The rates of resistance were intermediate, but genotype-guided therapy resulted in high rates of viral suppression. The observed resistance profile should not be an obstacle to the use of the dolutegravir-based regimen now recommended in Brazil, but genotype testing may be warranted before initiating first-generation NNRTI-based regimens.

Similar content being viewed by others
References
Günthard HF, Calvez V, Paredes R, Pillay D, Shafer RW, Wensing AM, Jacobsen DM, Richman DD (2018) Human Immunodeficiency Virus Drug Resistance: 2018 recommendations of the international antiviral society-USA Panel. Clin Infect Dis
Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D’Arminio Monforte A, Hermans P, Goebel FD, Blaxhult A, Kirk O, Phillips AN, EuroSIDA Study Group (2002) A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: Results from the EuroSIDA study. J Infect Dis 185:178–187
Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG (2017) Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 18:256–266
Hermankova M, Ray SC, Ruff C, Powell-Davis M, Ingersoll R, D’Aquila RT, Ingersoll R, D’Aquila RT, Quinn TC, Siliciano JD, Siliciano RF, Persaud D (2001) HIV-1 drug resistance profiles in children and adults with viral load of > 50 copies/ml receiving combination therapy. JAMA 286:196–207
Marschner IC, Collier AC, Coombs RW, D’Aquila RT, DeGruttola V, Fischl MA, Hammer SM, Hughes MD, Johnson VA, Katzenstein DA, Richman DD, Smeaton LM, Spector SA, Saag MS (1998) Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis 177:40–47
Bennett DE, Bertagnolio S, Sutherland D, Gilks CF (2008) The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther 13:1–13
Asboe D, Aitken C, Boffito M, Booth C, Cane P, Fakoya A et al (2011) BHIVA Guidelines Subcommittee et al British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals
Hoen B, Bonnet F, Delaugerre C, Delobel P, Goujard C, L’Hénaff M, Persiaux R, Rey D, Rouzioux C, Taburet AM, Morlat P French HIV expert group (2013) French 2013 guidelines for antiretroviral therapy of HIV-1 infection in adults. J Int AIDS Soc 17:19034
Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/guidelines. Accessed 17 July 2018
Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais.—Brasília: Ministério da Saúde, 2018. http://www.aids.gov.br/pt-br/pub/2013/protocolo-clinico-e-diretrizes-terapeuticas-para-manejo-da-infeccao-pelo-hiv-em-adultos. Accessed 17 Jan 2018
Teira R, Vidal F, Muñoz-Sánchez P, Geijo P, Viciana P, Ribera E, Domingo P, Castaño M, Martínez E, Roca B, Puig T, Estrada V, Deig E, Galindo MJ, de la Fuente B, Lozano F, Montero M, Muñoz-Sanz A, Sanchez T, Terrón A, Romero-Palacios A, Lacalle JR, Garrido M, Suárez-Lozano I, VACH Study Group (2017) Very low level viraemia and risk of virological failure in treated HIV-1-infected patients. HIV Med 18:196–203
Berta P, Marta G, Sonia P, Angelina C, Angeles C, Alvaro M, Iria RO, Andres T, Jose DP, Eva P (2016) Any impact of blips and low-level viraemia episodes among HIV-infected patients with sustained virological suppression on ART? J Antimicrob Chemother 71:1051–1055
Doyle T, Smith C, Vitiello P, Cambiano V, Johnson M, Owen A, Phillips AN, Geretti AM (2012) Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis 54:724–732
Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H (2013) Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 57:1489–1496
Hermans LE, Moorhouse M, Carmona S, Grobbee DE, Hofstra LM, Richman DD, Tempelman HA, Venter WDF, Wensing AMJ (2018) Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 18:188–197
Alencar CS, Sabino EC, Carvalho SM, Leao SC, Carneiro-Proietti AB, Capuani L, Oliveira CL, Carrick D, Birch RJ, Gonçalez TT, Keating S, Swanson PA, Hackett J Jr, Busch MP (2013) NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II), International Component. HIV genotypes and primary drug resistance among HIV-seropositive blood donors in Brazil: role of infected blood donors as sentinel populations for molecular surveillance of HIV. J Acquir Immune Defic Syndr 63:387–392
Ferreira JL, Rodrigues R, Lança AM, de Almeida VC, Rocha SQ, Ragazzo TG, Estevam DL, Brigido LF (2013) Transmitted drug resistance among people living with HIV/aids at major cities of Sao Paulo State, Brazil. Adv Virol 15:878237
Guimarães PM, Ferreira JL, Coelho LP, Cavalcanti Jde S, Lopes GI, Matsuda EM, Almeida FJ, Almeida VC, Campeas AE, Junior LC, Brígido LF, São Paulo Savage Workgroup (2015) Transmitted Drug Resistence Among Recently Diagnosed Adults and Children in São Paulo, Brazil. AIDS Res Hum Retroviruses 31:1219–1224
HIV Drug Resistance Report 2017. http://www.who.int/hiv
Arruda MB, Boullosa LT, Cardoso CC, da Costa CM, Alves CR, de Lima ST,Kaminski HT, Aleixo AW, Esposito AO, Cavalcanti AM, Riedel M, Couto-Fernandez JC, Ferreira SB, de Oliveira IC, Portal LE, Wolf HH, Fernandes SB, de M C Pardini MI, Feiteiro MV, Tolentino FM, Diaz RS, Lopes GI, Francisco RB, Véras NM, Pires AF, Franchini M, Mesquita F, Tanuri A HIV-BResNet Brazilian network for HIV Drug Resistance Surveillance (2018) (HIV-BresNet): a survey of treatment-naive individuals. J Int AIDS Soc 21
Wittkop L, Günthard HF, de Wolf F, Dunn D, Cozzi-Lepri A, de Luca A, Kücherer C, Obel N, von Wyl V, Masquelier B, Stephan C, Torti C, Antinori A, García F, Judd A, Porter K, Thiébaut R, Castro H, van Sighem AI, Colin C, Kjaer J, Lundgren JD, Paredes R, Pozniak A, Clotet B, Phillips A, Pillay D, Chêne G, EuroCoord-CHAIN study group (2011) Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 11:363–371
Margot NA, Wong P, Kulkarni R, White K, Porter D, Abram ME, Callebaut C, Miller MD (2017) Commonly transmitted HIV-1 drug resistance mutations in reverse transcriptase and protease in antiretroviral treatment-naive patients and response to regimens containing tenofovir disoproxil fumarate or tenofovir alafenamide. J Infect Dis 215:920–927
Zu Knyphausen F, Scheufele R, Kücherer C, Jansen K, Somogyi S, Dupke S, Jessen H, Schürmann D, Hamouda O, Meixenberger K, Bartmeyer B (2014) First line treatment response in patients with transmitted HIV drug resistance and well defined time point of HIV infection: updated results from the German HIV-1 seroconverter study. PLoS One. e95956
Clutter DS, Fessel WJ, Rhee SY, Hurley LB, Klein DB, Ioannidis JP, Silverberg MJ, Shafer RW (2016) Response to therapy in antiretroviral therapy-naive patients with isolated nonnucleoside reverse transcriptase inhibitor-associated transmitted drug resistance. J Acquir Immune Defic Syndr 72:171–176
Ashley O, Norbert B, Anders S, de Carmen M, Matthew P, Robert Z, Marie-Laure C, Maria P, Anne MBK, John G, Dimitrios P, Kholoud P, For CASCADE Collaboration in EuroCoordM (2018) Temporal trends of transmitted HIV drug resistance in a multinational seroconversion cohort. AIDS 32:161–169
http://www.unaids.org/sites/default/files/media_asset/2017-Global-AIDS-Monitoring_en.pdf. Accessed 17 Jan 2018
Avila-Rios S, Sued O, Rhee SY, Shafer RW, Reyes-Teran G, Ravasi G (2016) Surveillance of HIV transmitted drug resistance in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS One 29(11–16):e0158560
http://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf
Cesar C, Shepherd BE, Krolewiecki AJ, Fink VI, Schechter M, Tuboi SH, Wolff M, Pape JW, Leger P, Padgett D, Madero JS, Gotuzzo E, Sued O, McGowan CC, Masys DR, Cahn PE, Caribbean, Central and South America Network for HIV Research (CCASAnet) Collaboration of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Program (2018) Rates and reasons for early change of first HAART in HIV-1-infected patients in 7 sites throughout the Caribbean and Latin America. PLoS One 1:e0158560
Acknowledgements
We acknowledge the patients and their families, health care workers and laboratory personal from the participating sites, and data management personal.
MIHR: Monitoring Incident & Recent HIV infection working group: Daniela Rodrigues Colpas, Gabriela Bastos Cabral, Giselle Ibete Silva López Lopes, Norberto Camilo Campos, Isadora Coutinho Ribeiro, Cintia Mayumi Ahagon, Guilherme Penteado Teixeira, João Leandro de Paula Ferreira, Ivana Barros de Campos, Mariana Carvalho, Rafaella Gomes, Flavia Gennari Pinheiro, Luiz Carlos Pereira Jr, Karen Morejon, Álvaro Furtado da Costa, Érika Maria do Nascimento Kalmar, Fábio Luis Nascimento Nogui, Fábio Luis Nascimento Nogui, Patrícia Rady Müller, Silvia Pereira Goulart, Suzana Toledo da Silva Leme.
Funding
FAPESP (award number 2013/19441-7 and PPSUS 2016/14813-1).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were identified by the authors.
Ethical approval
The research involved no intervention on humans or animals, and patients in this observational study agreed to be included and signed an informed consent statement. Additional MIRH-participating institutions include Regional de Santo André do Instituto Adolfo Lutz /SP, Ambulatório de Moléstia Infecciosa, Jundiaí; Hospital das Clínicas da Faculdade de Medicina, Ribeirão Preto/SP; Instituto de Infectologia Emilio Ribas/SP.
Additional information
Handling Editor: Li Wu.
Rights and permissions
About this article
Cite this article
Coelho, L.P.O., Matsuda, E.M., Nogueira, R.S. et al. Prevalence of HIV-1 transmitted drug resistance and viral suppression among recently diagnosed adults in São Paulo, Brazil. Arch Virol 164, 699–706 (2019). https://doi.org/10.1007/s00705-018-04122-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-04122-8