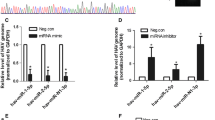
Abstract
Hepatitis C virus (HCV) is a worldwide threaten to human health with a high ratio of chronic infections. Recently, we found that Vpr-mediated regulation of HCV replication depends on the host protein DDB1-Cul4 associate factor 1 (DCAF1), implying that DCAF1 might be involved in the replication of HCV. In this study, we demonstrated that DCAF1 knockdown reduced HCV replication both in the infectious (JFH1) and replicon (Con1) systems. Further investigation showed a negative regulation of HCV internal ribosome entry site (IRES)-mediated translation by DCAF1. Considering the positive effects on the replication of the HCV replicon, we speculated that DCAF1 affected the balance between HCV RNA replication and protein translation. Since miR-122 is involved in the regulation of this balance, we investigated the influence of DCAF1 on miR-122 expression. By measuring the expression of miR-122, pre-miR-122 and its target CAT-1 mRNA, we found that miR-122 was downregulated following DCAF1 knockdown. Furthermore, overexpression of miR-122 rescued HCV replication impairment induced by DCAF1 knockdown. In conclusion, our study suggests that DCAF1 is involved in HCV replication through regulation of miR-122 and thus provides new insights into the interaction between HCV and the host cell.






Similar content being viewed by others
References
Ahn J, Vu T, Novince Z, Guerrero-Santoro J, Rapic-Otrin V, Gronenborn AM (2010) HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation. J Biol Chem 285:37333–37341
Ambros V (2001) microRNAs: tiny regulators with great potential. Cell 107:823–826
Casey Klockow L, Sharifi HJ, Wen X, Flagg M, Furuya AK, Nekorchuk M, de Noronha CM (2013) The HIV-1 protein Vpr targets the endoribonuclease dicer for proteasomal degradation to boost macrophage infection. Virology 444:191–202
Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM (2004) miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 1:106–113
Collier AJ, Tang S, Elliott RM (1998) Translation efficiencies of the 5’ untranslated region from representatives of the six major genotypes of hepatitis C virus using a novel bicistronic reporter assay system. J Gen Virol 79(Pt 10):2359–2366
Conrad KD, Giering F, Erfurth C, Neumann A, Fehr C, Meister G, Niepmann M (2013) MicroRNA-122 dependent binding of Ago2 protein to hepatitis C virus RNA is associated with enhanced RNA stability and translation stimulation. PloS One 8:e56272
Deng A, Chen C, Ishizaka Y, Chen X, Sun B, Yang R (2014) Human immunodeficiency virus type 1 Vpr increases hepatitis C virus RNA replication in cell culture. Virus Res 184:93–102
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H (2014) Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 61:S45–S57
Guo Z, Kong Q, Liu C, Zhang S, Zou L, Yan F, Whitmire JK, Xiong Y, Chen X, Wan YY (2016) DCAF1 controls T-cell function via p53-dependent and -independent mechanisms. Nat Commun 7:10307
Hajarizadeh B, Grebely J, Dore GJ (2013) Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 10:553–562
Han Q, Xu C, Wu C, Zhu W, Yang R, Chen X (2009) Compensatory mutations in NS3 and NS5A proteins enhance the virus production capability of hepatitis C reporter virus. Virus Res 145:63–73
Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M (2008) microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J 27:3300–3310
Jin J, Arias EE, Chen J, Harper JW, Walter JC (2006) A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23:709–721
Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309:1577–1581
Jopling CL, Schutz S, Sarnow P (2008) Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe 4:77–85
Kato T, Date T, Murayama A, Morikawa K, Akazawa D, Wakita T (2006) Cell culture and infection system for hepatitis C virus. Nat Protoc 1:2334–2339
Kaur M, Khan MM, Kar A, Sharma A, Saxena S (2012) CRL4-DDB1-VPRBP ubiquitin ligase mediates the stress triggered proteolysis of Mcm10. Nucleic Acids Res 40:7332–7346
Kim K, Heo K, Choi J, Jackson S, Kim H, Xiong Y, An W (2012) Vpr-binding protein antagonizes p53-mediated transcription via direct interaction with H3 tail. Mol Cell Biol 32:783–796
Kim K, Kim JM, Kim JS, Choi J, Lee YS, Neamati N, Song JS, Heo K, An W (2013) VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell 52:459–467
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12:735–739
Lavanchy D (2011) Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 17:107–115
Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM (2013) Competing and noncompeting activities of miR-122 and the 5’ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Nat Acad Sci USA 110:1881–1886
Li ZY, Xi Y, Zhu WN, Zeng C, Zhang ZQ, Guo ZC, Hao DL, Liu G, Feng L, Chen HZ, Chen F, Lv X, Liu DP, Liang CC (2011) Positive regulation of hepatic miR-122 expression by HNF4alpha. J Hepatol 55:602–611
Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R (1999) Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113
Ma L, Shen CJ, Cohen EA, Xiong SD, Wang JH (2014) miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP. PloS One 9:e99535
Masaki T, Arend KC, Li Y, Yamane D, McGivern DR, Kato T, Wakita T, Moorman NJ, Lemon SM (2015) miR-122 stimulates hepatitis C virus RNA synthesis by altering the balance of viral RNAs engaged in replication versus translation. Cell Host Microbe 17:217–228
McCall CM, Miliani de Marval PL, Chastain PD, Jackson SC, He YJ, Kotake Y, Cook JG, Xiong Y (2008) Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol Cell Biol 28:5621–5633
Nakagawa T, Mondal K, Swanson PC (2013) VprBP (DCAF1): a promiscuous substrate recognition subunit that incorporates into both RING-family CRL4 and HECT-family EDD/UBR5 E3 ubiquitin ligases. BMC Mol Biol 14:22
Rijnbrand R, Bredenbeek P, van der Straaten T, Whetter L, Inchauspe G, Lemon S, Spaan W (1995) Almost the entire 5’ non-translated region of hepatitis C virus is required for cap-independent translation. FEBS Lett 365:115–119
Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR (2005) Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol 79:10978–10987
Shimakami T, Yamane D, Jangra RK, Kempf BJ, Spaniel C, Barton DJ, Lemon SM (2012) Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc Nat Acad Sci USA 109:941–946
Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J (2008) Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog 4:e1000059
Thibault PA, Huys A, Dhillon P, Wilson JA (2013) MicroRNA-122-dependent and -independent replication of Hepatitis C Virus in Hep3B human hepatoma cells. Virology 436:179–190
Thibault PA, Huys A, Amador-Canizares Y, Gailius JE, Pinel DE, Wilson JA (2015) Regulation of hepatitis C virus genome replication by Xrn1 and MicroRNA-122 binding to individual sites in the 5’ untranslated region. J Virol 89:6294–6311
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M (2009) Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461:798–801
Wang L, Jeng KS, Lai MM (2011) Poly(C)-binding protein 2 interacts with sequences required for viral replication in the hepatitis C virus (HCV) 5’ untranslated region and directs HCV RNA replication through circularizing the viral genome. J Virol 85:7954–7964
Wen X, Casey Klockow L, Nekorchuk M, Sharifi HJ, de Noronha CM (2012) The HIV1 protein Vpr acts to enhance constitutive DCAF1-dependent UNG2 turnover. PloS One 7:e30939
Yan Y, Huang F, Yuan T, Sun B, Yang R (2016) HIV-1 Vpr increases HCV replication through VprBP in cell culture. Virus Res 223:153–160
Yoo BJ, Spaete RR, Geballe AP, Selby M, Houghton M, Han JH (1992) 5’ end-dependent translation initiation of hepatitis C viral RNA and the presence of putative positive and negative translational control elements within the 5’ untranslated region. Virology 191:889–899
Zhang S, Feng Y, Narayan O, Zhao LJ (2001) Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene 263:131–140
Zhou D, Wang Y, Tokunaga K, Huang F, Sun B, Yang R (2015) The HIV-1 accessory protein Vpr induces the degradation of the anti-HIV-1 agent APOBEC3G through a VprBP-mediated proteasomal pathway. Virus Res 195:25–34
Acknowledgements
We would like to thank Dr. Takaji Wakita (National Institute of Infectious Diseases, Japan) for providing the JFH1 plasmid, and Dr. F.V. Chisari (The Scripps Research Institute, USA) for providing the Huh7.5.1 cells. We are grateful for technical support from the Core Facility and Technical Support, Wuhan Institute of Virology. This work was funded by the Key National Science and Technology Program in the 12th Five-Year Period (Grant2012ZX10001-006) and the Key Laboratory on Emerging Infectious Disease and Biosafety in Wuhan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Michael Carpenter.
Rights and permissions
About this article
Cite this article
Yan, Y., Li, C., Sun, B. et al. DCAF1 is involved in HCV replication through regulation of miR-122. Arch Virol 163, 977–985 (2018). https://doi.org/10.1007/s00705-017-3691-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3691-8