Abstract
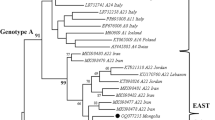
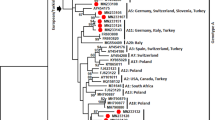
Small ruminant lentiviruses (SRLVs), which comprise caprine arthritis-encephalitis virus (CAEV) and maedi-visna virus (MVV), are prevalent in goats and sheep worldwide, including in Japan. However, little is known about the molecular characteristics of goat lentiviruses in Japan. In this study, a molecular and phylogenetic analysis of the long gag region was performed. The phylogenic tree demonstrated that all samples belonged to SRLV subtype B1. Two clusters were identified, with one cluster distinct from previously reported strains of subtype B1. In addition, several alterations in the amino acid sequence were detected in immunodominant epitopes of the gag region. To gain a deeper understanding of the genetic diversity of SRLVs in Japan, it will be necessary to increase the sample size and conduct a broader survey. The present report is important for establishing baseline information on the prevalence of SRLV in Japan and providing data to develop a new, more sensitive diagnostic test for effective control of SRLV.



Similar content being viewed by others
References
Ramírez H, Reina R, Amorena B et al (2013) Small ruminant lentiviruses: genetic variability, tropism and diagnosis. Viruses 5:1175–1207. doi:10.3390/v5041175
Minardi da Cruz J, Singh D, Lamara A, Chebloune Y (2013) Small Ruminant Lentiviruses (SRLVs) break the species barrier to acquire new host range. Viruses 5:1867–1884. doi:10.3390/v5071867
Leroux C, Cruz JCM, Mornex J-F (2010) SRLVs: a genetic continuum of lentiviral species in sheep and goats with cumulative evidence of cross species transmission. Curr HIV Res 8:94–100
Shah C, Böni J, Huder JB et al (2004) Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade. Virology 319:12–26. doi:10.1016/j.virol.2003.09.047
Li Y, Zhou F, Li X et al (2013) Development of TaqMan-based qPCR method for detection of caprine arthritis–encephalitis virus (CAEV) infection. Arch Virol 158:2135–2141. doi:10.1007/s00705-013-1728-1
Konishi M, Tsuduku S, Haritani M et al (2004) An epidemic of caprine arthritis encephalitis in Japan: isolation of the virus. J Vet Med Sci Jpn Soc Vet Sci 66:911–917
Oguma K, Tanaka C, Harasawa R et al (2014) Isolation of maedi/visna virus from a sheep in Japan. J Vet Med Sci Jpn Soc Vet Sci 76:211–218
Konishi M, Hiratani M, Kimura K et al (2007) Epidemiological survey and pathological studies on Caprine arthritis-encephalitis (CAE) in Japan. Bull Natl Inst Anim Health 113:23–30
Konishi M, Hayama Y, Shirafuji H et al (2016) Serological survey of caprine arthritis-encephalitis virus infection in Japan. J Vet Med Sci Jpn Soc Vet Sci 78:447–450. doi:10.1292/jvms.15-0357
Reina R, Berriatua E, Luján L et al (2009) Prevention strategies against small ruminant lentiviruses: an update. Vet J 182:31–37. doi:10.1016/j.tvjl.2008.05.008
Peterhans E, Greenland T, Badiola J et al (2004) Routes of transmission and consequences of small ruminant lentiviruses (SRLVs) infection and eradication schemes. Vet Res 35:257–274. doi:10.1051/vetres:2004014
de Andrés D, Klein D, Watt NJ et al (2005) Diagnostic tests for small ruminant lentiviruses. Vet Microbiol 107:49–62. doi:10.1016/j.vetmic.2005.01.012
Brinkhof J, van Maanen C (2007) Evaluation of five enzyme-linked immunosorbent assays and an agar gel immunodiffusion test for detection of antibodies to small ruminant lentiviruses. Clin Vaccine Immunol 14:1210–1214. doi:10.1128/CVI.00282-06
Giangaspero M, Osawa T, Orusa R et al (2011) Epidemiological survey for visna-maedi among sheep in northern prefectures of Japan. Vet Ital 47:437–451
Brajon G, Daniela M, Manuele L et al (2012) Development and field testing of a real-time PCR assay for caprine arthritis-encephalitis-virus (CAEV). Open Virol J 6:82–90. doi:10.2174/1874357901206010082
de Andrés X, Ramírez H, Bertolotti L et al (2013) An insight into a combination of ELISA strategies to diagnose small ruminant lentivirus infections. Vet Immunol Immunopathol 152:277–288. doi:10.1016/j.vetimm.2012.12.017
González B, Reina R, García I et al (2005) Mucosal immunization of sheep with a Maedi-Visna virus (MVV) env DNA vaccine protects against early MVV productive infection. Vaccine 23:4342–4352. doi:10.1016/j.vaccine.2005.03.032
Padiernos RBC, Balbin MM, Parayao AM, Mingala CN (2015) Molecular characterization of the gag gene of caprine arthritis encephalitis virus from goats in the Philippines. Arch Virol 160:969–978. doi:10.1007/s00705-015-2359-5
Leroux C, Vuillermoz S, Mornex J-F, Greenland T (1995) Genomic heterogeneity in the pol region of ovine lentiviruses obtained from bronchoalveolar cells of infected sheep from France. J Gen Virol 76:1533–1537. doi:10.1099/0022-1317-76-6-1533
Leroux C, Chastang J, Greenland T, Mornex JF (1997) Genomic heterogeneity of small ruminant lentiviruses: existence of heterogeneous populations in sheep and of the same lentiviral genotypes in sheep and goats. Arch Virol 142:1125–1137
Zanoni RG (1998) Phylogenetic analysis of small ruminant lentiviruses. J Gen Virol 79:1951–1961. doi:10.1099/0022-1317-79-8-1951
Narayan O, Wolinsky JS, Clements JE et al (1982) Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol 59:345–356. doi:10.1099/0022-1317-59-2-345
Narayan O, Kennedy-Stoskopf S, Sheffer D et al (1983) Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun 41:67–73
Gorrell MD, Brandon MR, Sheffer D et al (1992) Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocyte. J Virol 66:2679–2688
Guiguen F, Mselli-Lakhal L, Durand J et al (2000) Experimental infection of Mouflon-domestic sheep hybrids with caprine arthritis-encephalitis virus. Am J Vet Res 61:456–461
Larruskain A, Jugo B (2013) Retroviral infections in sheep and goats: small ruminant lentiviruses and host interaction. Viruses 5:2043–2061. doi:10.3390/v5082043
L’Homme Y, Ouardani M, Lévesque V et al (2011) Molecular characterization and phylogenetic analysis of small ruminant lentiviruses isolated from Canadian sheep and goats. Virol J 8:271. doi:10.1186/1743-422X-8-271
Shah C, Huder JB, Boni J et al (2004) Direct evidence for natural transmission of small-ruminant lentiviruses of subtype A4 from goats to sheep and vice versa. J Virol 78:7518–7522. doi:10.1128/JVI.78.14.7518-7522.2004
Fras M, Leboeuf A, Labrie F-M et al (2013) Phylogenetic analysis of small ruminant lentiviruses in mixed flocks: multiple evidence of dual infection and natural transmission of types A2 and B1 between sheep and goats. Infect Genet Evol 19:97–104. doi:10.1016/j.meegid.2013.06.019
Santry LA, de Jong J, Gold AC et al (2013) Genetic characterization of small ruminant lentiviruses circulating in naturally infected sheep and goats in Ontario, Canada. Virus Res 175:30–44. doi:10.1016/j.virusres.2013.03.019
Grego E, Profiti M, Giammarioli M et al (2002) Genetic heterogeneity of small ruminant lentiviruses involves immunodominant epitope of capsid antigen and affects sensitivity of single-strain-based immunoassay. Clin Diagn Lab Immunol 9:828–832
Rosati S, Mannelli A, Merlo T, Ponti N (1999) Characterization of the immunodominant cross-reacting epitope of visna maedi virus and caprine arthritis-encephalitis virus capsid antigen. Virus Res 61:177–183
Tang S, Zhao J, Wang A et al (2010) Characterization of immune responses to capsid protein p24 of human immunodeficiency virus type 1 and implications for detection. Clin Vaccine Immunol CVI 17:1244–1251. doi:10.1128/CVI.00066-10
Mammano F, Ohagen A, Höglund S, Göttlinger HG (1994) Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J Virol 68:4927–4936
Freed EO (1998) HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251:1–15. doi:10.1006/viro.1998.9398
Provitera P, Goff A, Harenberg A et al (2001) Role of the major homology region in assembly of HIV-1 Gag. Biochemistry (Mosc) 40:5565–5572
Purdy JG, Flanagan JM, Ropson IJ et al (2008) Critical role of conserved hydrophobic residues within the major homology region in mature retroviral capsid assembly. J Virol 82:5951–5961. doi:10.1128/JVI.00214-08
Chang Y-F, Wang S-M, Huang K-J, Wang C-T (2007) Mutations in capsid major homology region affect assembly and membrane affinity of HIV-1 Gag. J Mol Biol 370:585–597. doi:10.1016/j.jmb.2007.05.020
Fluri A, Nenci C, Zahno M-L et al (2006) The MHC-haplotype influences primary, but not memory, immune responses to an immunodominant peptide containing T- and B-cell epitopes of the caprine arthritis encephalitis virus Gag protein. Vaccine 24:597–606. doi:10.1016/j.vaccine.2005.08.043
Gamble TR, Vajdos FF, Yoo S et al (1996) Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285–1294
Gamble TR, Yoo S, Vajdos FF et al (1997) Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849–853
Wernike K, Hoffmann B, Kalthoff D et al (2011) Development and validation of a triplex real-time PCR assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J Virol Methods 174:77–84. doi:10.1016/j.jviromet.2011.03.028
Fieni F, Rowe J, Van Hoosear K et al (2002) Presence of caprine arthritis-encephalitis virus (CAEV) infected cells in flushing media following oviductal-stage embryo collection. Theriogenology 57:931–940
Acknowledgement
This study was supported by the Research Project for Improving Food Safety and Animal Health of the Ministry of Agriculture, Forestry, and Fisheries of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The procedures for blood sample collection from goats were carried out based on the guidelines for animal care and use of Tokyo University of Agriculture and Technology.
Rights and permissions
About this article
Cite this article
Kokawa, S., Oba, M., Hirata, T. et al. Molecular characteristics and prevalence of small ruminant lentiviruses in goats in Japan. Arch Virol 162, 3007–3015 (2017). https://doi.org/10.1007/s00705-017-3447-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3447-5