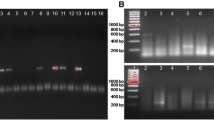
Abstract
Dengue disease is caused by dengue viruses 1-4 and has been ranked by the World Health Organisation (WHO) as the fastest spreading vector-borne viral disease. Dengue is often underreported and misdiagnosed due to a wide spectrum of clinical manifestations. Diagnosis of dengue is based on clinical case definitions and laboratory methods. Newer case definitions of dengue have been formulated by clinical studies in order to improve case detection. Owing to its epidemic potential, mortality and morbidity, there is a need for a rapid and accurate diagnostic assay for dengue in order to help the clinician in the early detection of cases and to prevent disease progression. A duplex real time PCR targeting the 3’UTR region for rapid and simultaneous detection of all dengue viruses serotypes (1-4) was standardized based on published literature. About 150 patients with acute undifferentiated febrile illness classified based on the 2009 WHO dengue case definition were tested using the duplex real time dengue PCR. Sequencing based PCR was performed on selected PCR positive samples for partial nucleotide sequence of the CprM gene and a phylogenetic tree was constructed. Statistical analysis was done using the MedCalc software. Out of the 126 patients classified as dengue disease positive, according to the 2009 WHO dengue case definition, 54% had “probable dengue”, 43% had “dengue with warning signs” and 3% had “severe dengue”. The performance of the duplex real time PCR was assessed among the various clinical groups of dengue and it was found that in the “dengue with warning signs group” PCR had a positive predictive value of 85.29% (range - 68.94% to 95.05%) when compared with dengue NS1 ELISA. The average time for PCR positivity was found to be four days from the onset of illness. The cycling threshold values obtained from real time PCR were used as a semi quantitative measure of viremia. Accordingly, there was a relatively low CT value among the “warning signs dengue group” when compared to the “probable dengue group”. The use of the duplex PCR is suggested in the early diagnosis of dengue, especially in the ‘warning signs’ group of patients as they showed a higher positivity rate. Also, the use of the resultant CT value as a semi-quantitative measure of viremia will assist the clinician in early diagnosis and prevention of disease development.





Similar content being viewed by others
References
World Health Organisation WHO (2012) Global strategy for dengue prevention and control 2012–2020
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O (2013) The global distribution and burden of dengue. Nature 496:504–507
Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ (2006) The WHO dengue classification and case definitions: time for a reassessment. Lancet 368:170–173
Callahan JD, Wu S-JL, Dion-Schultz A, Mangold BE, Peruski LF, Watts DM, Porter KR, Murphy GR, Suharyono W, King C-C (2001) Development and evaluation of serotype-and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J Clin Microbiol 39:4119–4124
World Health Organization WHO, Research SPf, Diseases TiT, Diseases WHODoCoNT, Epidemic WHO, Alert P (2009) Dengue: guidelines for diagnosis, treatment, prevention and control
Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG (2010) Evaluation of diagnostic tests: dengue. Nat Rev Microbiol 8:S30–S37
De Paula SO, Fonseca BALd (2004) Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz J Infect Dis 8:390–398
Kosasih H, Alisjahbana B, Widjaja S, de Mast Q, Parwati I, Blair PJ, Burgess TH, van der Ven A, Williams M (2013) The diagnostic and prognostic value of dengue non-structural 1 antigen detection in a hyper-endemic region in Indonesia. PLoS One 8:e80891
Kassim FM, Izati MN, TgRogayah T, Apandi YM, Saat Z (2011) Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J Trop Med Public Health 42:562
Duong V, Ly S, Try PL, Tuiskunen A, Ong S, Chroeung N, Lundkvist A, Leparc-Goffart I, Deubel V, Vong S (2011) Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Negl Trop Dis 5:e1244
Blacksell SD, Mammen MP, Thongpaseuth S, Gibbons RV, Jarman RG, Jenjaroen K, Nisalak A, Phetsouvanh R, Newton PN, Day NP (2008) Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagnos Microbiol Infect Dis 60:43–49
Lanciotti RS, Calisher CH, Gubler DJ, Chang G-J, Vorndam AV (1992) Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 30:545–551
Narayanan M, Aravind M, Thilothammal N, Prema R, Sargunam CR, Ramamurty N (2002) Dengue fever epidemic in Chennai—a study of clinical profile and outcome. Indian Pediatr 39:1027–1033
Macedo GA, Gonin MLC, Pone SM, Cruz OG, Nobre FF, Brasil P (2014) Sensitivity and specificity of the World Health Organization dengue classification schemes for severe dengue assessment in children in Rio de Janeiro. PLoS One 9:e96314
Mishra B, Singhal L, Sethi S, Ratho R (2013) Leptospirosis coexistent with dengue fever: a diagnostic dilemma. J Global Infect Dis 5:121
Parker TM, Murray CK, Richards AL, Samir A, Ismail T, Fadeel MA, Jiang J, Wasfy MO, Pimentel G (2007) Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hyg 77:390–392
Lee I-K, Liu J-W, Yang KD (2005) Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg 72:221–226
Basuki PS (2003) Concurrent dengue infection and enteric fever. A case series. Folia Med Indones 39:54
Bhalla A, Sharma N, Sharma A, Suri V (2006) Concurrent infection with dengue and malaria. Indian J Med Sci 60
Kalawat U, Sharma K, Reddy S (2011) Prevalence of dengue and chickungunya fever and their co-infection. Indian J Pathol Microbiol 54:844
Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA (2000) Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181:2–9
Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP (2011) Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis 5:e1309
Erra EO, Korhonen EM, Voutilainen L, Huhtamo E, Vapalahti O, Kantele A (2013) Dengue in travellers: kinetics of viremia and NS1 antigenemia and their associations with clinical parameters. PLoS One 8:e65900
Gupta N, Srivastava S, Jain A, Chaturvedi UC (2012) Dengue in India. Indian J Med Res 136:373
Gupta E, Mohan S, Bajpai M, Choudhary A, Singh G (2012) Circulation of Dengue virus-1 (DENV-1) serotype in Delhi, during 2010–11 after Dengue virus-3 (DENV-3) predominance: a single centre hospital-based study. J Vector Borne Dis 49:82
Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, Kabra SK, Broor S (2008) Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J 5:1
Bhatia R, Dash AP, Sunyoto T (2013) Changing epidemiology of dengue in South-East Asia. WHO South-East Asia J Public Health 2:23
Dar L, Gupta E, Narang P, Broor S (2006) Cocirculation of dengue serotypes, Delhi, India, 2003. Emerg Infect Dis 12:352
Anoop M, Issac A, Mathew T, Philip S, Kareem NA, Unnikrishnan R, Sreekumar E (2010) Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India
Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, Ramos C, Rico-Hesse R (1999) Dengue virus structural differences that correlate with pathogenesis. J Virol 73:4738–4747
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Indian Council of Medical Research (ICMR).
Conflict of interest
The authors declare that there was no conflict of interest.
Ethical approval
This study was approved by the Institutional ethics committee (IEC-NI/12/OCT/30/50).Informed consent from adults and assent form from children aged (12–18) was obtained prior to sample collection.
Authors’ contributions
VS: made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data and drafting the manuscript. GS: made substantial contributions to conception and design acquisition of data, or analysis and interpretation of data. KS: made substantial contributions for analysis and interpretation of data. KK: made substantial contributions analysis and interpretation of data and critical revision of intellectual content. GP: made substantial contributions critical revision of intellectual content and final approval of the version to be published. PS: made substantial contributions conception and design, analysis and interpretation of data and drafting the manuscript, critical revision of intellectual content and final approval of the version to be published.
Rights and permissions
About this article
Cite this article
Seshan, V., Sarangan, G., Sheriff, K. et al. Serological, molecular and clinical correlates of dengue from a tertiary care centre in Chennai, India. Arch Virol 162, 2983–2988 (2017). https://doi.org/10.1007/s00705-017-3429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3429-7