Abstract
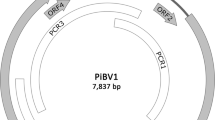
Piry virus (PIRYV) is a rhabdovirus (genus Vesiculovirus) and is described as a possible human pathogen, originally isolated from a Philander opossum trapped in Para State, Northern Brazil. This study describes the complete full coding sequence and the genetic characterization of PIRYV. The genome sequence reveals that PIRYV has a typical vesiculovirus-like organization, encoding the five genes typical of the genus. Phylogenetic analysis confirmed that PIRYV is most closely related to Perinet virus and clustered in the same clade as Chandipura and Isfahan vesiculoviruses.


Similar content being viewed by others
References
Brun G, Bao X, Prevec L (1995) The relationship of Piry virus to other vesiculoviruses: a re-evaluation based on the glycoprotein gene sequence. Intervirology 38:274–282
Cherian SS, Gunjikar RS, Banerjee A, Kumar S, Arankalle VA (2012) Whole genomes of Chandipura virus isolates and comparative analysis with other rhabdoviruses. PLoS One 7:e30315
Clerc Y, Rodhain F, Digoutte JP, Tesh R, Heme G, Coulanges P (1982) The Perinet virus, rhabdoviridae, of the vesiculovirus type isolated in Madagascar from Culicidae. Arch Inst Pasteur Madagascar 49:119–129
de Sousa AA, Reis R, Bento-Torres J, Trevia N, Lins NA, Passos A, Santos Z, Diniz JA, Vasconcelos PF, Cunningham C, Perry VH, Diniz CW (2011) Influence of enriched environment on viral encephalitis outcomes: behavioral and neuropathological changes in albino Swiss mice. PLoS One 6:e15597
Figueiredo LT, da Rosa AP, Fiorillo AM (1985) Prevalence of neutralizing antibodies to Piry arbovirus in subjects of the region of Ribeirao Preto, State of Sao Paulo. Rev Inst Med Trop Sao Paulo 27:157–161
Gurav YK, Tandale BV, Jadi RS, Gunjikar RS, Tikute SS, Jamgaonkar AV, Khadse RK, Jalgaonkar SV, Arankalle VA, Mishra AC (2010) Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res 132:395–399
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Lichty BD, Power AT, Stojdl DF, Bell JC (2004) Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med 10:210–216
Marriott AC (2005) Complete genome sequences of Chandipura and Isfahan vesiculoviruses. Arch Virol 150:671–680
McCluskey BJ, Mumford EL (2000) Vesicular stomatitis and other vesicular, erosive, and ulcerative diseases of horses. Vet Clin North Am Equine Pract 16:457–469 (viii–ix)
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274
Nichol ST, Holland JJ (1987) Genome RNA terminus conservation and diversity among vesiculoviruses. J Virol 61:200–205
Peng Y, Leung HC, Yiu SM, Chin FY (2012) IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28:1420–1428
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Pinheiro FP, Bensabath G, Andrade AH, Lins ZC, Fraiha H, Tang AT, Lainson R, Shaw JJ, Azevedo MC (1974) Infectious diseases along Brazil’s trans-amazon highway: surveillance and research. Bull Pan Am Health Organ 8:111–122
Tavares-Neto J, Travassos da Rosa AP, Ataide M, Morais-Souza H, Vasconcelos P, Travassos da Rosa J (1990) Frequency of neutralizing antibodies to the vesiculovirus Piry, in blood donors of Uberaba, Minas Gerais, Brazil. Rev Inst Med Trop Sao Paulo 32:211–214
Tesh R, Saidi S, Javadian E, Loh P, Nadim A (1977) Isfahan virus, a new vesiculovirus infecting humans, gerbils, and sandflies in Iran. Am J Trop Med Hyg 26:299–306
Vasilakis N, Widen S, Travassos da Rosa AP, Wood TG, Walker PJ, Holmes EC, Tesh RB (2013) Malpais spring virus is a new species in the genus vesiculovirus. Virol J 10:69
Walker PJ, Firth C, Widen SG, Blasdell KR, Guzman H, Wood TG, Paradkar PN, Holmes EC, Tesh RB, Vasilakis N (2015) Evolution of genome size and complexity in the rhabdoviridae. PLoS Pathog 11:e1004664
Wilks CR, House JA (1984) Susceptibility of various animals to the vesiculovirus Piry. J Hyg (Lond) 93:147–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (Grant No. 13/14929-1 and 14/02438-6, and Scholarships No. 12/24150-9; 15/05778-5; 14/20851-8, 13/02256-2). MRTN is supported by the CNPq (Grant Nos. 302032/2011-8 and 200024/2015-9).
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Multiple sequence alignment of conserved polymerase gene (L) domains I through VI of vesiculoviruses. Alignments were performed using MAFFT and edited manually. Numbers represent genome positions in Piry vesiculovirus. (TIFF 5520 kb)
Supplementary Figure 2
Multiple sequence alignment of conserved pre-motifs A and motifs A to D of vesiculoviruses. Alignments were performed using MAFFT and edited manually. Numbers represent genome positions in Piry vesiculovirus. (TIFF 11271 kb)
Rights and permissions
About this article
Cite this article
de Souza, W.M., Acrani, G.O., Romeiro, M.F. et al. Complete genome sequence of Piry vesiculovirus. Arch Virol 161, 2325–2328 (2016). https://doi.org/10.1007/s00705-016-2905-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-016-2905-9