Abstract
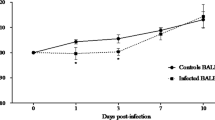
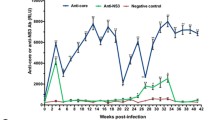
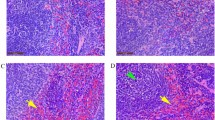
Oxidative stress is a disturbance in the oxidant-antioxidant balance leading to potential cellular damage. Most cells can tolerate a mild degree of oxidative stress because they have a system that counteracts oxidation that includes antioxidant molecules such as glutathione (GSH) and superoxide dismutase (SOD). Disruption of the host antioxidant status has been recognized as an important contributor to the pathogenesis of many viruses. Caraparu virus (CARV) is a member of group C of the Bunyaviridae family of viruses. In South American countries, group C bunyaviruses are among the common agents of human febrile illness and have caused multiple notable outbreaks of human disease in recent decades; nevertheless, little is known about the pathogenic characteristics of these viruses. The purpose of this study was to examine the hepatic pathogenesis of CARV in mice and the involvement of oxidative stress and antioxidant defenses on this pathology. Following subcutaneous infection of BALB/c mice, CARV was detected in the liver, and histopathology revealed acute hepatitis. Increased serum levels of aspartate and alanine aminotransferases (AST/ALT) and greater hepatic expression of the proinflammatory cytokine tumor necrosis factor-α (TNF-α) were found in infected animals. CARV infection did not alter the biomarkers of oxidative stress but caused an increase in GSH content and altered the expression and activity of SOD. This is the first report of an alteration of oxidative homeostasis upon CARV infection, which may, in part, explain the hepatic pathogenesis of this virus, as well as the pathogenesis of other Bunyaviridae members.









Similar content being viewed by others
References
Afonso V, Santos G, Collin P, Khatib AM, Mitrovic DR, Lomri N, Leitman DC, Lomri A (2006) Tumor necrosis factor-alpha down-regulates human Cu/Zn superoxide dismutase 1 promoter via JNK/AP-1 signaling pathway. Free Radic Biol Med 41:709–721
Bernardes-Terzian AC, de-Moraes-Bronzoni RV, Drumond BP, da Silva-Nunes M, da Silva NS, Urbano-Ferreira M, Sperança MA, Nogueira ML (2009) Sporadic oropouche virus infection, Acre, Brazil. Emerg Infect Dis 15:348–350
Brinton MA, Gavin EI, Lo WK, Pinto AJ, Morahan OS (1993) Characterization of murine Caraparu Bunyavirus liver infection and imunomodulator-mediated antiviral protection. Antiviral Res 20:155–171
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP (2003) Inhibition of influenza infection by glutathione. Free Radic Biol Med 34:928–936
Casals J, Whitman L (1961) Group C, a new serological group of hitherto undescribed arthropod-borne viruses. Immunological studies. Am J Trop Med Hyg 10:250–258
Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP (2001) Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J Biol Chem 276:19715–19722
Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A (2006) Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 174:1361–1369
Causey OR, Causey CE, Maroja OM, Macedo DG (1961) The isolation of arthropod-born viruses including members of two hitherto undescribed serological groups, in the Amazon Region of Brazil. Am J Trop Med Hyg 10:227–249
Chen TH, Tang P, Yang CF, Kao LH, Lo YP, Chuang CK, Shih YT, Chen WJ (2011) Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology 410:410–417
de Brito Magalhães CL, Drumond BP, Novaes RF, Quinan BR, de Magalhães JC, dos Santos JR, Pinto CA, Assis MT, Bonjardim CA, Kroon EG, Ferreira PC (2011) Identification of a phylogenetically distinct orthobunyavirus from group C. Arch Virol 156:1173–1184
Elliott RM, Weber F (2009) Bunyaviruses and the Type I interferon system. Viruses 1:1003–1021
Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, Vallejo E, Madrid C, Aguayo N, Gotuzzo E, Suarez V, Morales AM, Beingolea L, Reyes N, Perez J, Negrete M, Rocha C, Morrison AC, Russell KL, Blair PJ, Olson JG, Kochel TJ (2010) Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl Trop Dis 4:787
Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K (2000) Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 376:1–13
Ha HL, Shin HJ, Feitelson MA, Yu DY (2010) Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 16:6035–6043
Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP (2011) Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 183:1550–1560
Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A (2009) Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol 41:348–357
Huang SH, Cao XJ, Liu W, Shi XY, Wei W (2010) Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res 48:109–116
Iversson LB, Travassos da Rosa APA, Coimbra TLM, Ferreira IB, Nassar ES (1987) Human disease in Ribeira Valley, Brazil caused by Caraparu, a group C arbovirus- report of a case. Rev Inst Med Trop 29:112–117
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A (2004) Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 279:2461–2469
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356
Narayanan A, Popova T, Turell M, Kidd J, Chertow J, Popov SG, Bailey C, Kashanchi F, Kehn-Hall K (2011) Alteration in superoxide dismutase 1 causes oxidative stress and p38 MAPK activation following RVFV infection. PLoS One 6:e20354
Nunes MRT, Travassos da Rosa APA, Weaver SC, Tesh RB, Vasconcelos PF (2005) Molecular epidemiology of group C viruses (Bunyaviridae, Orthobunyavirus) isolated in the Americas. J Virol 79:10561–10570
Ostergaard H, Tachibana C, Winther JR (2004) Monitoring disulfide bond formation in the eukaryotic cytosol. J Cell Biol 166:337–345
Palamara AT, Garaci E, Rotilio G, Ciriolo MR, Casabianca A, Fraternale A, Rossi L, Schiavano GF, Chiarantini L, Magnani M (1996) Inhibition of murine AIDS by reduced glutathione. AIDS Res Hum Retroviruses 12:1373–1381
Palamara AT, Perno CF, Ciriolo MR, Dini L, Balestra E, D’Agostini C, Di Francesco P, Favalli C, Rotilio G, Garaci E (1995) Evidence for antiviral activity of glutathione: in vitro inhibition of herpes simplex virus type 1 replication. Antiviral Res 27:237–253
Papi A, Contoli M, Gasparini P, Bristot L, Edwards MR, Chicca M, Leis M, Ciaccia A, Caramori G, Johnston SL, Pinamonti S (2008) Role of xanthine oxidase activation and reduced glutathione depletion in rhinovirus induction of inflammation in respiratory epithelial cells. J Biol Chem 283:28595–28606
Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, Ishak R, Freitas RB, Gomes ML, LeDuc JW, Oliva OF (1981) Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am J Trop Med Hyg 30:149–160
Qin Y, Tian Y (2010) Protective effects of total glucosides of paeony and the underlying mechanisms in carbon tetrachloride-induced experimental liver injury. Arch Med Sci 7:604–612
Schmaljohn CS, Nichol ST (2007) Bunyaviridae. In: Fields BN, Knipe DM, Howley PM, Griffin DE (eds) Fields Virology, 5th edn. Lippincott Williams and Wilkins, Philadelphia, pp 1741–1778
Shope RE, Whitman L (1966) Nepuyo virus, a new group C agent isolated in Trinidad and Brazil. II Serological studies. Am J Trop Med Hyg 15:772–774
Shope RE, Woodall JP, Travassos da Rosa APA (1988) The epidemiology of disease caused by viruses in group C and Guamá (Bunyaviridae). In: Monath TP (ed) The arboviruses: epidemiology and ecology. CRC Press Inc, Boca Raton, pp 37–52
Soldan SS, González-Scarano F (2005) Emerging infectious diseases: the Bunyaviridae. J Neurovirol 11:412–423
Tian Y, Jiang W, Gao N, Zhang J, Chen W, Fan D, Zhou D, An J (2010) Inhibitory effects of glutathione on dengue virus production. Biochem Biophys Res Commun 397:420–424
Vasconcelos HB, Azevedo RS, Casseb SM, Nunes-Neto JP, Chiang JO, Cantuária PC, Segura MN, Martins LC, Monteiro HA, Rodrigues SG, Nunes MR, Vasconcelos PF (2009) Oropouche fever epidemic in Northern Brazil: epidemiology and molecular characterization of isolates. J Clin Virol 44:129–133
Vasconcelos PFC, Da Rosa JF, Da Rosa AP, Degallier N, Pinheiro FP, Sá Filho GC (1991) Epidemiology of encephalitis caused by arbovirus in the Brazilian Amazonia. Rev Inst Med Trop Sao Paulo 33:465–476
Wang J, Chen Y, Gao N, Wang Y, Tian Y, Wu J, Zhang J, Zhu J, Fan D, An J (2013) Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS One 8:e55407
Xiong Q, Xie P, Li H, Hao L, Li G, Qiu T, Liu Y (2010) Acute effects of microcystins exposure on the transcription of antioxidant enzyme gene in three organs (liver, kidney, and testis) of male Wistar rats. J Biochem Mol Toxicol 24:361–367
Zamocky M, Furtmuller PG, Obinger C (2008) Evolution of catalases from bacteria to humans. Antioxid Redox Signal 10:1527–1548
Acknowledgements
This work received financial support from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) – Process APQ-04125-10, Brazil. We thank Universidade Federal de Ouro Preto (UFOP) and the Research Center in Biological Sciences (NUPEB/UFOP), Brazil. The authors are grateful to colleagues from the Virus Laboratory (UFMG), Laboratory of Metabolic Biochemistry (UFOP) and Experimental Nutrition (UFOP) for their technical and scientific support. We are also grateful to Maria Terezinha Bahia from Chagas’ Disease Laboratory (UFOP) for the use of the real-time PCR ABI 7300 equipment (Applied Biosystems) and Jaquelline G. de Oliveira, Marcelo Eustáquio Silva, Melina Oliveira de Souza and Joamyr Victor Rossoni Junior for help with certain experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camini, F.C., Almeida, L.T., Bernardes, C.S. et al. Caraparu virus induces damage and alterations in antioxidant defenses in the liver of BALB/c mice after subcutaneous infection. Arch Virol 159, 2621–2632 (2014). https://doi.org/10.1007/s00705-014-2123-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2123-2