Abstract
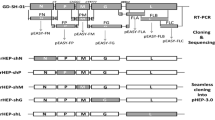
The aG rabies virus strain has been attenuated through multiple passages in cells and is now used as a vaccine strain in China. We attempted to develop a reverse genetics system using the aG strain. Recombinant full-length genomic cDNA was flanked by a hammerhead ribozyme and the hepatitis delta virus ribozyme. Three helper plasmids encoding the nucleoprotein, the phosphoprotein, and the large protein were produced and introduced together with a plasmid containing the full-length aG viral genome into BHK-21 cells by transfection. Recombinant virus was successfully recovered from the cloned cDNA under the control of a CMV promoter driven by RNA polymerase II. The recombinant virus was confirmed by RT-PCR, and the titer of the recombinant virus was 6.2 log LD50.



Similar content being viewed by others
References
Ito N, Takayama M, Yamada K, Suqiyama M, Minamoto N (2001) Rescue of rabies virus from cloned cDNA and identification of the pathogenicity-related gene: glycoprotein gene is associated with virulence for adult mice. J Virol 75(19):9121–9128
Huang Y, Tang Q, Nadin-Davis SA et al (2010) Development of a reverse genetics system for a human rabies virus vaccine strain employed in China. Virus Res 149:28–35
Garcia-Sastre A (1998) Negative-strand RNA viruses: application to biotechnology. Trends Biotechnol 16:230–235
Schnell MJ, Mebatsion T, Conzelmann KK (1994) Infectious rabies viruses from cloned cDNA. EMBO J 13(18):4195–4203
Lawson ND, Stillman EA, Whitt MA, Rose JK (1995) Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA 92(10):4477–4481
Whelan SP, Ball LA, Barr JN, Wertz GT (1995) Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA 92(18):8388–8392
Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter MA (1995) Rescue of measles viruses from cloned DNA. EMBO J 14(23):5773–5784
Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR (1995) Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA 92(25):11563–11567
Garcin D, Pelet T, Calain P, Roux L, Curran J, Kolakofsky D (1995) A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: genetation of a novel copy-back nondefective interfering virus. EMBO J 14(24):6087–6094
Kato A, Sakai Y, Shioda T, Kondo T, Nakanishi M, Naqai Y (1996) Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells 1(6):569–579
Ito N, Takayama-Ito M, Yamada K, Hosokawa J, Suqiyama M, Minamoto N (2003) Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol Immunol 47(8):613–617
Le Mercier P, Jacob Y, Tanner K, Tordo N (2002) A novel expression cassette of lyssavirus show that the distantly related Mokola virus can rescue a defective rabies virus genome. J Virol 76:2024–2027
Sigurdsson ST, Eckstein F (1995) Structure-function relationships of hammerhead ribozyme: from understanding to applications. Trends Biotechnol 13:286–289
Been MD, Wickham GS (1997) Self-cleaving ribozyme of hepatitis delta virus RNA. Eur J Biochem 247:741–753
Inoue KI, Shoji Y, Kurane I, Iijima T, Sakai T, Morimoto K (2003) An improved method for recovering rabies virus from cloned cDNA. J Virol Methods 107(2):229–236
Wenqiang J, Xiangping Y, Zhiyong L, Xi L, Xuerui L, Xiaoting T, Baoyu L, Liu J (2011) Molecular characterization of China rabies virus vaccine strain. Virol J 8:521
Wu X, Rupprecht CE (2008) Glycoprotein gene relocation in rabies virus. Virus Res 131(1):95–99
Tao L, Ge J, Wang X, Zhai H, Hua T, Zhao B, Kong D, Yang C, Chen H, Bu Z (2010) Molecular basis of neurovirulence of flury rabies virus vaccine strains: importance of the polymerase and the glycoprotein R333Q mutation. J Virol 84(17):8926–8936
Dietzschold B, Wunner WH, Wiktor TJ, Lopes AD, Lafon M, Smith CL, Koprowski H (1983) Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA 80(1):70–74
Tuffereau C, Leblois H, Benejran J, Coulon P, Lafay F, Flamand A (1989) Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology 172(1):206–212
Faber M, Faber ML, Papaneri A, Bette M, Weihe E, Dietzschold B, Schnell MJ (2005) A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol 79(22):14141–14148
Seif I, Coulon P, Rollin PE, Flamand A (1985) Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol 53(3):926–934
McGettigan JP, Foley HD, Belyakov IM, Berzofsky JA, Pomerntz RJ, Schnell MJ (2001) Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelop protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelop proteins from different HIV-1 isolates. J Virol 75(9):4430–4434
Du J, Tang Q, Huang Y, Rodney WE, Wang L, Liang G (2011) Development of recombinant rabies virus vectors with Gaussia luciferase reporter based on Chinese vaccine strain CTN181. Virus Res 160(1–2):82–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wenqiang, J., Yin, X., Lan, X. et al. Development of a reverse genetics system for the aG strain of rabies virus in China. Arch Virol 159, 1033–1038 (2014). https://doi.org/10.1007/s00705-013-1919-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1919-9