Abstract
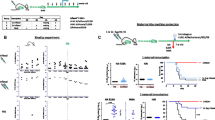
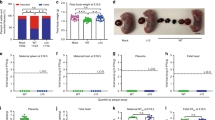
H5N1 influenza virus is one of the viruses that can potentially cause an influenza pandemic. Protection of newborns against influenza virus infection could be effectively provided by maternal immunization. In this study, female mice were immunized with H5N1 HA DNA vaccine or inactivated whole-virion vaccine, and the protection provided by maternal antibodies in their offspring against a lethal homologous influenza virus challenge was compared. The results showed that maternal antibodies, whether induced by a DNA vaccine or an inactivated vaccine, could completely protect offspring aged 1-4 weeks from a lethal influenza virus challenge. Breast-feeding was the major route of transfer for maternal antibodies. Milk-derived antibodies were able to effectively protect the offspring aged 1-4 weeks from lethal influenza virus infection, whereas maternal antibodies transferred through the placenta only partially protected the offspring 1-2 weeks of age. The milk- and placenta-transferred IgG2a antibody levels in offspring from their mothers, whether vaccinated with DNA vaccine or inactivated vaccine, were higher than the IgG1 levels. Our results indicated that maternal vaccination with HA DNA, as well as with whole-virion inactivated vaccine, could offer effective protection to offspring against H5N1 influenza virus infection.



Similar content being viewed by others
References
Aihara H, Miyazaki J (1998) Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 16:867–870
Bailhache M, Sarlangue J, Castella C, Richer O, Fleury H, Koeck JL (2011) Influenza A(H1N1)v virus infection in infants less than 6 months of age in southwestern France. Arch Pediatr 18:383–389
Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY (2005) Avian influenza A (H5N1) infection in humans. N Engl J Med 353:1374–1385
Bender JM, Ampofo K, Gesteland P, Sheng X, Korgenski K, Raines B, Daly JA, Valentine K, Srivastava R, Pavia AT, Byington CL (2010) Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J 29:6–9
Beyer WE, Palache AM, Osterhaus AD (1998) Comparison of serology and reactogenicity between influenza subunit vaccines and whole virus or split vaccines: a review and meta-analysis of the literature. Clin Drug Investig 15:1–12
Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC (2006) Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657–1664
Chen J, Zhang F, Fang F, Chang H, Chen Z (2007) Vaccination with hemagglutinin or neuraminidase DNA protects BALB/c mice against influenza virus infection in presence of maternal antibody. BMC Infect Dis 7:118
Chen Z, Sahashi Y, Matsuo K, Asanuma H, Takahashi H, Iwasaki T, Suzuki Y, Aizawa C, Kurata T, Tamura S (1998) Comparison of the ability of viral protein-expressing plasmid DNAs to protect against influenza. Vaccine 16:1544–1549
Chen Z, Kadowaki S, Hagiwara Y, Yoshikawa T, Matsuo K, Kurata T, Tamura S (2000) Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18:3214–3222
Chen Z (2004) Influenza DNA vaccine: an update. Chin Med J (Engl) 117:125–132
Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, Lochindarat S, Srisan P, Suwan P, Osotthanakorn Y, Anantasetagoon T, Kanjanawasri S, Tanupattarachai S, Weerakul J, Chaiwirattana R, Maneerattanaporn M, Poolsavathitikool R, Chokephaibulkit K, Apisarnthanarak A, Dowell SF (2005) Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 11:201–209
Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG (1998) Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472–477
Cohen J (1997) The flu pandemic that might have been. Science 277:1600–1601
de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL (1997) A pandemic warning? Nature 389:554
Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, O’Brien KL (2011) Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med 165:104–111
Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, Renshaw M, Sambhara S, Katz JM (2002) DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis 8:796–801
Feltquate DM, Heaney S, Webster RG, Robinson HL (1997) Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol 158:2278–2284
Gall SA (2005) Maternal immunization to protect the mother and neonate. Expert Rev Vaccines 4:813–818
Geeraedts F, Bungener L, Pool J, ter Veer W, Wilschut J, Huckriede A (2008) Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respi Viruses 2:41–51
Gendon IuZ (2009) Prevention of influenza in pregnant women and neonatal infants. Vopr Virusol 54:4–10
Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB (2004) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 53:1–40
Hoare M, Levy MS, Bracewell DG, Doig SD, Kong S, Titchener-Hooker N, Ward JM, Dunnill P (2005) Bioprocess engineering issues that would be faced in producing a DNA vaccine at up to 100 m3 fermentation scale for an influenza pandemic. Biotechnol Prog 21:1577–1592
Hovden AO, Cox RJ, Haaheim LR (2005) Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand J Immunol 62:36–44
Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA (2006) Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 13:981–990
Hwang SD, Shin JS, Ku KB, Kim HS, Cho SW, Seo SH (2010) Protection of pregnant mice, fetuses and neonates from lethality of H5N1 influenza viruses by maternal vaccination. Vaccine 28:2957–2964
Jakeman KJ, Smith H, Sweet C (1989) Mechanism of immunity to influenza: maternal and passive neonatal protection following immunization of adult ferrets with a live vaccinia-influenza virus haemagglutinin recombinant but not with recombinants containing other influenza virus proteins. J Gen Virol 70(Pt 6):1523–1531
Kim JH, Jacob J (2009) DNA vaccines against influenza viruses. Curr Top Microbiol Immunol 333:197–210
Kitikoon P, Nilubol D, Erickson BJ, Janke BH, Hoover TC, Sornsen SA, Thacker EL (2006) The immune response and maternal antibody interference to a heterologous H1N1 swine influenza virus infection following vaccination. Vet Immunol Immunopathol 112:117–128
Kodihalli S, Goto H, Kobasa DL, Krauss S, Kawaoka Y, Webster RG (1999) DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol 73:2094–2098
Krief WI, Levine DA, Platt SL, Macias CG, Dayan PS, Zorc JJ, Feffermann N, Kuppermann N (2009) Influenza virus infection and the risk of serious bacterial infections in young febrile infants. Pediatrics 124:30–39
Kumagai T, Nagai K, Okui T, Tsutsumi H, Nagata N, Yano S, Nakayama T, Okuno Y, Kamiya H (2004) Poor immune responses to influenza vaccination in infants. Vaccine 22:3404–3410
Li X, Fang F, Song Y, Yan H, Chang H, Sun S, Chen Z (2006) Essential sequence of influenza neuraminidase DNA to provide protection against lethal viral infection. DNA Cell Biol 25:197–205
Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, Gao Q, Zhang Z, Liu Y, Wang Z, Yang M, Sun R, Li C, Lin S, Ji M, Wang X, Wood J, Feng Z, Wang Y, Yin W (2006) Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368:991–997
Lopez-Medina E, Ardura MI, Siegel JD, Brock E, Sanchez PJ (2011) 2009 Influenza A in Infants hospitalized at younger than 6 months. J Pediatr 160:626–631
Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y (2004) Failure of inactivated influenza A vaccine to protect healthy children aged 6–24 months. Pediatr Int 46:122–125
Mbawuike IN, Six HR, Cate TR, Couch RB (1990) Vaccination with inactivated influenza A virus during pregnancy protects neonatal mice against lethal challenge by influenza A viruses representing three subtypes. J Virol 64:1370–1374
Mesonero A, Suarez DL, van Santen E, Tang DC, Toro H (2011) Avian influenza in ovo vaccination with replication defective recombinant adenovirus in chickens: vaccine potency, antibody persistence, and maternal antibody transfer. Avian Dis 55:285–292
Newman JO (1960) Transmission of antibodies against influenza virus A across the placenta. J Coll Gen Pract 3:434–440
Nicholson KG, Tyrrell DA, Harrison P, Potter CW, Jennings R, Clark A, Schild GC, Wood JM, Yetts R, Seagroatt V, Huggins A, Anderson SG (1979) Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J Biol Stand 7:123–136
Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y (2010) High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J Virol 84:10918–10922
Pertmer TM, Roberts TR, Haynes JR (1996) Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol 70:6119–6125
Saito S (2000) Cytokine network at the feto-maternal interface. J Reprod Immunol 47:87–103
Sauerbrei A, Schmidt-Ott R, Hoyer H, Wutzler P (2009) Seroprevalence of influenza A and B in German infants and adolescents. Med Microbiol Immunol 198:93–101
Shin JS, Kim HS, Cho SH, Seo SH (2010) IgG antibodies mediate protective immunity of inactivated vaccine for highly pathogenic H5N1 influenza viruses in ferrets. Viral Immunol 23:321–327
Steel J (2011) New strategies for the development of H5N1 subtype influenza vaccines: progress and challenges. BioDrugs 25:285–298
Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC (2003) Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet 362:1959–1966
Sugimura T, Ito Y, Tananari Y, Ozaki Y, Maeno Y, Yamaoka T, Kudo Y (2008) Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine 26:2700–2705
Sweet C, Jakeman KJ, Smith H (1987) Role of milk-derived IgG in passive maternal protection of neonatal ferrets against influenza. J Gen Virol 68(Pt 10):2681–2686
Tamura D, Miura T, Uehara R, Sugaya N (2005) Dosage of inactivated influenza vaccine for infants. Kansenshogaku Zasshi 79:427–432
Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O’Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM (2001) Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19:1732–1737
Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M (2006) Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 354:1343–1351
Tsuda H, Michimata T, Hayakawa S, Tanebe K, Sakai M, Fujimura M, Matsushima K, Saito S (2002) A Th2 chemokine, TARC, produced by trophoblasts and endometrial gland cells, regulates the infiltration of CCR4 + T lymphocytes into human decidua at early pregnancy. Am J Reprod Immunol 48:1–8
Vajo Z, Kosa L, Visontay I, Jankovics M, Jankovics I (2007) Inactivated whole virus influenza A (H5N1) vaccine. Emerg Infect Dis 13:807–808
Van de Perre P (2003) Transfer of antibody via mother’s milk. Vaccine 21:3374–3376
van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T (2006) H5N1 virus attachment to lower respiratory tract. Science 312:399
Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG (2000) A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA + CCR4 + lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol 115:640–646
WHO (2002) WHO manual on animal influenza diagnosis and surveillance. http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf
WHO (2012) Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2012 http://www.who.int/influenza/human_animal_interface/EN_GIP_20120116CumulativeNumberH5N1cases.pdf
Wilson WD, Mihalyi JE, Hussey S, Lunn DP (2001) Passive transfer of maternal immunoglobulin isotype antibodies against tetanus and influenza and their effect on the response of foals to vaccination. Equine Vet J 33:644–650
Wu J, Wang F, Fang F, Zhang W, Chang H, Zheng L, Chen Z (2011) Superior protection provided by a single dose of MF59-adjuvanted whole inactivated H5N1 influenza vaccine in type 1 diabetic mice. Arch Virol 156:387–395
Xu X, Subbarao, Cox NJ, Guo Y (1999) Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15–19
Yamaguchi K, Hisano M, Isojima S, Irie S, Arata N, Watanabe N, Kubo T, Kato T, Murashima A (2009) Relationship of Th1/Th2 cell balance with the immune response to influenza vaccine during pregnancy. J Med Virol 81:1923–1928
Yen CJ, Louie JK, Schechter R (2012) Infants hospitalized in intensive care units with 2009 H1N1 influenza infection, California, 2009–2010. Pediatr Infect Dis J 31:e52–e55
Zhang F, Chen J, Fang F, Zhou Y, Wu J, Chang H, Zhang R, Wang F, Li X, Wang H, Ma G, Chen Z (2005) Maternal immunization with both hemagglutinin- and neuraminidase-expressing DNAs provides an enhanced protection against a lethal influenza virus challenge in infant and adult mice. DNA Cell Biol 24:758–765
Zheng L, Wang F, Yang Z, Chen J, Chang H, Chen Z (2009) A single immunization with HA DNA vaccine by electroporation induces early protection against H5N1 avian influenza virus challenge in mice. BMC Infect Dis 9:17
Zhou Y, Fang F, Chen J, Wang H, Chang H, Yang Z, Chen Z (2008) Electroporation at low voltages enables DNA vaccine to provide protection against a lethal H5N1 avian influenza virus challenge in mice. Intervirology 51:241–246
Acknowledgments
This study was supported by National Natural Science Foundation of China (Project No. 30972623, 81071346) and National High Technology Research and Development Program of China (863 Program 2010AA022905, 2010AA022908, 2010AA022910).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, F., Fang, F., Chang, H. et al. Comparison of protection against H5N1 influenza virus in mouse offspring provided by maternal vaccination with HA DNA and inactivated vaccine. Arch Virol 158, 1253–1265 (2013). https://doi.org/10.1007/s00705-013-1621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1621-y