Abstract
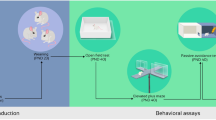
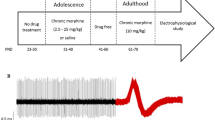
Opioid abuse during pregnancy may have noteworthy effects on the child’s behavioral, emotional and cognitive progression. In this study, we assessed the effect of prenatal exposure to morphine on electrophysiological features of locus coeruleus (LC) noradrenergic neurons which is involved in modulating cognitive performance. Pregnant dams were randomly divided into two groups, that is a prenatal saline treated and prenatal morphine-treated group. To this end, on gestational days 11–18, either morphine or saline (twice daily, s.c.) was administered to pregnant dams. Whole-cell patch-clamp recordings were conducted on LC neurons of male offspring. The evoked firing rate, instantaneous frequency and action potentials half-width, and also input resistance of LC neurons significantly increased in the prenatal morphine group compared to the saline group. Moreover, action potentials decay slope, after hyperpolarization amplitude, rheobase current, and first spike latency were diminished in LC neurons following prenatal exposure to morphine. In addition, resting membrane potential, rise slope, and amplitude of action potentials were not changed by prenatal morphine exposure. Together, the current findings show a significant enhancement in excitability of the LC neurons following prenatal morphine exposure, which may affect the release of norepinephrine to other brain regions and/or cognitive performances of the offspring.




Similar content being viewed by others
References
Aghajanian GK (1978) Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature 276:186–188
Ahmadi-Soleimani SM, Azizi H, Gompf HS, Semnanian S (2017a) Role of orexin type-1 receptors in paragiganto-coerulear modulation of opioid withdrawal and tolerance: a site specific focus. Neuropharmacology 126:25–37
Alaee E, Moazen P, Pattij T, Semnanian S, Azizi H (2021) Prenatal exposure to morphine impairs attention and impulsivity in adult rats. Psychopharmacology 238:2729–2741
Ali AB, Bannister AP, Thomson AM (2007) Robust correlations between action potential duration and the properties of synaptic connections in layer 4 interneurones in neocortical slices from juvenile rats and adult rat and cat. J Physiol 580:149–169
Amaral DG, Foss JA (1975) Locus coeruleus lesions and learning. Science 188:377–378
Arima J, Kubo C, Ishibashi H, Akaike N (1998) α2-Adrenoceptor-mediated potassium currents in acutely dissociated rat locus coeruleus neurones. J Physiol 508:57
Arnsten AFT (1999) Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast 7:133–146
Aston-Jones G, Bloom FE (1981) Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900
Aston-Jones G, Cohen JD (2005) Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J Comp Neurol 493:99–110
Aston-Jones G, Shipley MT, Chouvet G, Ennis M, Van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Bl A (1991) Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res 88:47–75
Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T (1994) Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci 14:4467–4480
Bayer SA (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Bean BP (2007) The action potential in mammalian central neurons. Nat Rev Neurosci 8:451–465
Benarroch EE (2009) The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73:1699–1704
Berridge CW, Waterhouse BD (2003) The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42:33–84
Bird SJ, Kuhar MJ (1977) Iontophoretic application of opiates to the locus coeruleus. Brain Res 122:523–533
Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP (2004) Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24:5301–5306
Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM (2011) Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res 218:200–205
Chandler DJ, Gao W-J, Waterhouse BD (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci 111:6816–6821
Chiou L-C, Yeh G-C, Fan S-H, How C-H, Chuang K-C, Tao P-L (2003) Prenatal morphine exposure decreases analgesia but not K+ channel activation. NeuroReport 14:239–242
Christie MJ (1991) Mechanisms of opioid actions on neurons of the locus coeruleus. Prog Brain Res 88:197–205
Davis CP, La’Tonya MF, Johnson GS, Schrott LM (2010) Prenatal oxycodone exposure impairs spatial learning and/or memory in rats. Behav Brain Res 212:27–34
Elsworth JD, Morrow BA, Nguyen V-T, Mitra J, Picciotto MR, Roth RH (2007) Prenatal cocaine exposure enhances responsivity of locus coeruleus norepinephrine neurons: role of autoreceptors. Neuroscience 147:419–427
Faber ESL, Sah P (2003) Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9:181–194
Farahani F, Azizi H, Janahmadi M, Seutin V, Semnanian S (2021) Formalin-induced inflammatory pain increases excitability in locus coeruleus neurons. Brain Res Bull 172:52–60
Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci 77:3033–3037
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Forsythe ID, Linsdell P, Stanfield PR (1992) Unitary A-currents of rat locus coeruleus neurones grown in cell culture: rectification caused by internal Mg2+ and Na+. J Physiol 451:553–583
Guo H, Enters EK, McDowell KP, Robinson SE (1990) The effect of prenatal exposure to methadone on neurotransmitters in neonatal rats. Dev Brain Res 57:296–298
He HJ, Hnatczuk OC, Vathy I (1997) Exposure to morphine during gestation alters catecholaminergic immunoreactivity in the hypothalamus and the locus coeruleus of adult male rats. Soc Neurosci Abstr, pp 2148
Henderson G, Pepper CM, Shefner SA (1982) Electrophysiological properties of neurones contained in the locus coeruleus and mesencephalic nucleus of the trigeminal nerve in vitro. Exp Brain Res 45:29–37
Inoue M, Nakajima S, Nakajima Y (1988) Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol 407:177–198
Insel PA (1993) Adrenergic receptors, G proteins, and cell regulation: implications for aging research. Exp Gerontol 28:341–348
Ishimatsu M, Williams JT (1996) Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci 16:5196–5204
Kaeidi A, Azizi H, Javan M, Ahmadi SSM, Fathollahi Y, Semnanian S (2015) Direct facilitatory role of paragigantocellularis neurons in opiate withdrawal-induced hyperactivity of rat locus coeruleus neurons: an in vitro study. PLoS ONE 10:e0134873
Karimi SA, Hosseinmardi N, Sayyah M, Hajisoltani R, Janahmadi M (2021) Enhancement of intrinsic neuronal excitability-mediated by a reduction in hyperpolarization-activated cation current (Ih) in hippocampal CA1 neurons in a rat model of traumatic brain injury. Hippocampus 31:156–169
Khachaturian H, Alessi NE, Munfakh N, Watson SJ (1983) Ontogeny of opioid and related peptides in the rat CNS and pituitary: an immunocytochemical study. Life Sci 33:61–64
Kiritoshi T, Sun H, Ren W, Stauffer SR, Lindsley CW, Conn PJ, Neugebauer V (2013) Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology 66:170–178
Koob GF, Maldonado R, Stinus L (1992) Neural substrates of opiate withdrawal. Trends Neurosci 15:186–191
Kostrzewa RM, Klisans-Fuenmayor D (1984) Development of an opioid-specific action of morphine in modifying recovery of neonatally-damaged noradrenergic fibers in rat brain. Res Commun Chem Pathol Pharmacol 46:3–11
Kshatri AS, Gonzalez-Hernandez A, Giraldez T (2018) Physiological roles and therapeutic potential of Ca2+ activated potassium channels in the nervous system. Front Mol Neurosci 11:258
Lancaster B, Nicoll RA (1987) Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389:187–203
Levitt P, Moore RY (1979) Development of the noradrenergic innervation of neocortex. Brain Res 162:243–259
Loren I, Björklund A, Lindvall O (1976) The catecholamine systems in the developing rat brain: improved visualization by a modified glyoxylic acid-formaldehyde method. Brain Res 117:313–318
Loughlin SE, Foote SL, Fallon JH (1982) Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res Bull 9:287–294
Loy R, Moore RY (1979a) Ontogeny of the noradrenergic innervation of the rat hippocampal formation. Anat Embryol 157:243–253
Loy R, Moore RY (1979b) Regenerative growth of sympathetic fibers into rat hippocampus, Catecholamines: basic and clinical frontiers. Elsevier, Amsterdam, pp 1336–1338
Maeda T, Dresse A (1969) On the development of the locus ceruleus. I. Study of catecholamines with the fluorescent microscope. Acta Neurol Psychiatr Belg 69:5–10
Marshall KC, Christie MJ, Finlayson PG, Williams JT (1991) Developmental aspects of the locus coeruleus-noradrenaline system. Prog Brain Res 88:173–185
Masuko S, Nakajima Y, Nakajima S, Yamaguchi K (1986) Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci 6:3229–3241
Matschke LA, Rinné S, Snutch TP, Oertel WH, Dolga AM, Decher N (2018) Calcium-activated SK potassium channels are key modulators of the pacemaker frequency in locus coeruleus neurons. Mol Cell Neurosci 88:330–341
McGinty JF, Ford DH (1980) Effects of prenatal methadone on rat brain catecholamines. Dev Neurosci 3:224–234
Meng X, Lu Q, Rinzel J (2011) Control of firing patterns by two transient potassium currents: leading spike, latency, bistability. J Comput Neurosci 31:117–136
Mohell N, Svatengren J, Cannon B (1983) Identification of [3H] prazosin binding sites in crude membranes and isolated cells of brown adipose tissue as α1-adrenergic receptors. Eur J Pharmacol 92:15–25
Molineux ML, Fernandez FR, Mehaffey WH, Turner RW (2005) A-type and T-type currents interact to produce a novel spike latency-voltage relationship in cerebellar stellate cells. J Neurosci 25:10863–10873
Nichols CG, Lopatin AN (1997) Inward rectifier potassium channels. Annu Rev Physiol 59:171–191
Nieber K, Sevcik J, Illes P (1995) Hypoxic changes in rat locus coeruleus neurons in vitro. J Physiol 486:33–46
Niu L, Cao B, Zhu H, Mei B, Wang M, Yang Y, Zhou Y (2009) Impaired in vivo synaptic plasticity in dentate gyrus and spatial memory in juvenile rats induced by prenatal morphine exposure. Hippocampus 19:649–657
Nomura Y (2001) Neurophysiology of Rett syndrome. Brain Develop 23:S50–S57
Obstetricians American College of Gynecologists (2017) Committee opinion no. 711: opioid use and opioid use disorder in pregnancy. Obstet Gynecol 130:e81–e94
O’Callaghan JP, Holtzman SG (1976) Prenatal administration of morphine to the rat: tolerance to the analgesic effect of morphine in the offspring. J Pharmacol Exp Ther 197:533–544
Olson L, Seiger Å (1972) Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch 137:301–316
Ornoy A (2003) The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett 140:171–181
O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB (1994) Characterization of [3H] RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther 268:1362–1367
Osmanović SS, Shefner SA (1993) Calcium-activated hyperpolarizations in rat locus coeruleus neurons in vitro. J Physiol 469:89–109
Pachenari N, Azizi H, Semnaniann S (2019) Adolescent morphine exposure in male rats alters the electrophysiological properties of locus coeruleus neurons of the male offspring. Neuroscience 410:108–117
Peters MA, Turnbow M, Buchenauer D (1972) The distribution methadone treatment. J Pharmacol Exp Ther 181:273–278
Rasmussen K (1991) Afferent effects on locus coeruleus in opiate withdrawal. Prog Brain Res 88:207–216
Reisert I, Pilgrim C (1991) Sexual differentiation of monoaminergic neurons-genetic or epigenetic? Trends Neurosci 14:468–473
Riley MA, Vathy I (2006) Mid-to late gestational morphine exposure does not alter the rewarding properties of morphine in adult male rats. Neuropharmacology 51:295–304
Robinson SE, Mo Q, Maher JR, Wallace MJ, Kunko PM (1996) Perinatal exposure to methadone affects central cholinergic activity in the weanling rat. Drug Alcohol Depend 41:119–126
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223
Shah NS, Donald AG (1979) Pharmacological effects and metabolic fate of levo-methadone during postnatal development in rat. J Pharmacol Exp Ther 208:491–497
Shao L-R, Halvorsrud R, Borg-Graham L, Storm JF (1999) The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521:135
Shiekhattar R, Aston-Jones G (1994) Activation of adenylate cyclase attenuates the hyperpolarization following single action potentials in brain noradrenergic neurons independently of protein kinase A. Neuroscience 62:523–529
Sklair-Tavron L, Segal M (1993) Neurotrophic effects of cAMP generating systems on central noradrenergic neurons. Brain Res 614:257–269
Šlamberová R, Schindler CJ, Pometlová M, Urkuti C, Purow-Sokol JA, Vathy I (2001) Prenatal morphine exposure differentially alters learning and memory in male and female rats. Physiol Behav 73:93–103
Šlamberová R, Rimanóczy A, Bar N, Schindler CJ, Vathy I (2003) Density of μ-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus 13:461–471
Šlamberová R, Rimanóczy Á, Cao D, Schindler CJ, Vathy I (2005) Alterations of prenatal morphine exposure in μ-opioid receptor density in hypothalamic nuclei associated with sexual behavior. Brain Res Bull 65:479–485
Sldair-Tavron L, Nestler EJ (1995) Opposing effects of morphine and the neurotrophins, NT-3, NT-4, and BDNF, on locus coeruleus neurons in vitro. Brain Res 702:117–125
Soleimani SMA, Azizi H, Pachenari N, Mirnajafi-Zadeh J, Semnanian S (2017) Enhancement of μ-opioid receptor desensitization by orexin-A in rat locus coeruleus neurons. Neuropeptides 63:28–36
Stocker M, Hirzel K, D’hoedt D, Pedarzani P, (2004) Matching molecules to function: neuronal Ca2+-activated K+ channels and afterhyperpolarizations. Toxicon 43:933–949
Storm JF (1987) Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385:733–759
Storm JF (1990) Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83:161–187
Sugiyama D, Hur SW, Pickering AE, Kase D, Kim SJ, Kawamata M, Imoto K, Furue H (2012) In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J Physiol 590:2225–2231
Suto T, Eisenach JC, Hayashida K-I (2014) Peripheral nerve injury and gabapentin, but not their combination, impair attentional behavior via direct effects on noradrenergic signaling in the brain. PAIN® 155:1935–1942
Swanson LW (1976) The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res 110:39–56
Topley J, Windsor D, Williams R (2008) Behavioural, developmental and child protection outcomes following exposure to Class A drugs in pregnancy. Child Care Health Dev 34:71–76
Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K (2002) G-protein-gated potassium channels containing Kir3. 2 and Kir3. 3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22:4328–4334
Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM (2017) Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology 113:271–280
Vathy I (2002) Prenatal opiate exposure: long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology 27:273–283
Vathy I, Katay L (1992) Effects of prenatal morphine on adult sexual behavior and brain catecholamines in rats. Dev Brain Res 68:125–131
Vathy I, He H-J, Iodice M, Hnatczuk OC, Rimanóczy A (2000) Prenatal morphine exposure differentially alters TH-immunoreactivity in the stress-sensitive brain circuitry of adult male and female rats. Brain Res Bull 51:267–273
Vathy I, He HJ, Iodice M, Rimanóczy A, Gestational morphine alters the density of catecholamine fibers in the hypothalamus of adult female rats. Soc Neurosci Abstr, 1996, pp. 170.
Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R (2004) SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci 24:3537–3542
Wheeler DB, Randall A, Tsien RW (1996) Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci 16:2226–2237
Williams JT, North RA (1984) Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol 26:489–497
Williams JT, North RA, Shefner SA, Nishi S, Egan TM (1984) Membrane properties of rat locus coeruleus neurones. Neuroscience 13:137–156
Williams JT, Bobker DH, Harris GC (1991) Synaptic potentials in locus coeruleus neurons in brain slices. Prog Brain Res 88:167–172
Wilson GS, McCreary R, Kean J, Baxter JC (1979) The development of preschool children of heroin-addicted mothers: a controlled study. Pediatrics 63:135–141
Yamada M, Inanobe A, Kurachi Y (1998) G protein regulation of potassium ion channels. Pharmacol Rev 50:723–757
Yamamoto T, Ishikawa M, Tanaka C (1977) Catecholaminergic terminals in the developing and adult rat cerebellum. Brain Res 132:355–361
Zagon IS, Wu Y, McLaughlin PJ (1999) Opioid growth factor and organ development in rat and human embryos. Brain Res 839:313–322
Zhang X-F, Gopalakrishnan M, Shieh C-C (2003) Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience 122:1003–1011
Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C (2010) Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. Am J Physiol Cell Physiol 298:C635–C646
Zhu Y, Hsu M-S, Pintar JE (1998) Developmental expression of the μ, κ, and δ opioid receptor mRNAs in mouse. J Neurosci 18:2538–2549
Acknowledgements
We are enormously thankful to Dr. Tommy Pattij (Department of Anatomy and Neurosciences, Amsterdam University Medical Centers) and Dr. Narges Pachenari (Department of Physiology, Tarbiat Modares University) for beneficial comments and editing the manuscript. In addition, we would like to thank the financial support of the Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alaee, E., Farahani, F., Semnanian, S. et al. Prenatal exposure to morphine enhances excitability in locus coeruleus neurons. J Neural Transm 129, 1049–1060 (2022). https://doi.org/10.1007/s00702-022-02515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02515-3