Abstract
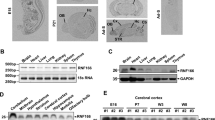
Ubiquitination and sumoylation are two important posttranslational modifications in cells. RING (Really Interesting New Gene)-type E3 ligases play essential roles in regulating a plethora of biological processes such as cell survival and death. In our previous study, we performed a microarray using inputs from MN9D dopaminergic neuronal cells treated with 6-hydroxydopamine and identified a novel RING-type E3 ligase, RNF166. We showed that RNF166 exerts proapoptotic effects via ubiquitin-dependent degradation of X-linked inhibitor of apoptosis and subsequent overactivation of caspase-dependent neuronal death following 6-hydroxydopamine treatment. In the present study, we further expanded the list of RNF166’s binding substrates using mass spectral analyses of immunoprecipitates obtained from RNF166-overexpressing HEK293 cells. Poly (ADP-ribose) polymerase 1, ATPase WRNIP1, X-ray repair cross-complementing protein 5 (Ku80), and replication protein A 70 were identified as potential binding partners of RNF166. Additionally, we confirmed that RNF166 interacts with and forms lysine 63-linked polyubiquitin chains in Ku80. Consequently, these events promoted the increased stability of Ku80. Intriguingly, we found that RNF166 also contains distinct consensus sequences termed SUMO-interacting motifs and interacts with apoptosis signal-regulating kinase 1 (ASK1). We determined that RNF166 induces the sumoylation of ASK1. Overall, our data provide novel evidence that RNF166 has a dual function of Lys63-linked ubiquitination and sumoylation of its cellular targets.




Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Bailly V, Lauder S, Prakash S, Prakash L (1997) Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J Biol Chem 272:23360–23365. https://doi.org/10.1074/jbc.272.37.23360
Bouchard VJ, Rouleau M, Poirier GG (2003) PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol 31:446–454. https://doi.org/10.1016/s0301-472x(03)00083-3
Boulton SJ, Jackson SP (1998) Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J 17:1819–1828. https://doi.org/10.1093/emboj/17.6.1819
Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243:1576–1583. https://doi.org/10.1126/science.2538923
Chen ZJ, Sun LJ (2009) Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 33:275–286. https://doi.org/10.1016/j.molcel.2009.01.014
Chu Y, Yang X (2011) SUMO E3 ligase activity of TRIM proteins. Oncogene 30:1108–1116. https://doi.org/10.1038/onc.2010.462
Cooper HJ, Tatham MH, Jaffray E, Heath JK, Lam TT, Marshall AG, Hay RT (2005) Fourier transform ion cyclotron resonance mass spectrometry for the analysis of small ubiquitin-like modifier (SUMO) modification: identification of lysines in RanBP2 and SUMO targeted for modification during the E3 autoSUMOylation reaction. Anal Chem 77(19):6310–6319. https://doi.org/10.1021/ac058019d
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252. https://doi.org/10.1016/s0092-8674(00)00116-1
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351–361. https://doi.org/10.1016/s0092-8674(00)00126-4
Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78:399–434. https://doi.org/10.1146/annurev.biochem.78.101807.09380
Desterro JM, Thomson J, Hay RT (1997) Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett 417:297–300. https://doi.org/10.1016/s0014-5793(97)01305-7
Dikic I, Wakatsuki S, Walters KJ (2009) Ubiquitin-binding domains—from structures to functions. Nat Rev Mol Cell Biol 10:659–671. https://doi.org/10.1038/nrm2767
Fell V, Schild-Poulter C (2014) The Ku heterodimer: function in DNA repair and beyond. Mutat Res Rev Mutat Res 763:15–29. https://doi.org/10.1016/j.mrrev.2014.06.002
Feng L, Chen J (2012) The E3 ligase RNF8 regulates KU80 removal and NHEJ repair. Nat Struct Mol Biol 19:201–206. https://doi.org/10.1038/nsmb.2211
Freemont PS, Hanson IM, Trowsdale J (1991) A novel cysteine-rich sequence motif. Cell 64:483–484. https://doi.org/10.1016/0092-8674(91)90229-r
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428. https://doi.org/10.1152/physrev.00027.2001
Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET (1997) Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem 272:28198–28201. https://doi.org/10.1074/jbc.272.45.28198
Gong L, Millas S, Maul GG, Yeh ET (2000) Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J Biol Chem 275:3355–3359. https://doi.org/10.1074/jbc.275.5.335
Hay RT (2005) SUMO: a history of modification. Mol Cell 18:1–12. https://doi.org/10.1016/j.molcel.2005.03.012
Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I (2006) Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem 281:16117–16127. https://doi.org/10.1074/jbc.M512757200
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479. https://doi.org/10.1146/annurev.biochem.67.1.425
Hochstrasser M (1996) Ubiquitin-dependent protein degradation. Annu Rev Genet 30:405–439. https://doi.org/10.1146/annurev.genet.30.1.405
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135–141. https://doi.org/10.1038/nature00991
Hurley JH, Lee S, Prag G (2006) Ubiquitin-binding domains. Biochem J 399:361–372. https://doi.org/10.1042/BJ20061138
Ivanov AV, Peng H, Yurchenko V, Yap KL, Negorev DG, Schultz DC, Psulkowski E, Fredericks WJ, White DE, Maul GG, Sadofsky MJ, Zhou MM, Rauscher FJ 3rd (2007) PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol Cell 28(5):823–837. https://doi.org/10.1016/j.molcel.2007.11.012
Jackson SP, Durocher D (2013) Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell 49:795–807. https://doi.org/10.1016/j.molcel.2013.01.017
Jacobson AD, Zhang NY, Xu P, Han KJ, Noone S, Peng J, Liu CW (2009) The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26 s proteasome. J Biol Chem 284:35485–35494. https://doi.org/10.1074/jbc.M109.052928
Johnson ES, Blobel G (1997) Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem 272:26799–26802
Kai M (2016) Kai, M. Roles of RNA-binding proteins in DNA damage response. Int. J. Mol. Sci. 2016, 17, 310. Int J Mol Sci 21:604. https://doi.org/10.3390/ijms17040604
Kanungo J (2016) DNA-PK deficiency in alzheimer’s disease. J Neurol Neuromed 1:17–22. https://doi.org/10.29245/2572.942x/2016/3.1016
Kerscher O (2007) SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep 8:550–555. https://doi.org/10.1038/sj.embor.7400980
Lee YS, Jang MS, Lee JS, Choi EJ, Kim E (2005) SUMO-1 represses apoptosis signal-regulating kinase 1 activation through physical interaction and not through covalent modification. EMBO Rep 6:949–955. https://doi.org/10.1038/sj.embor.7400511
Lelong IH, Petegnief V, Rebel G (1992) Neuronal cells mature faster on polyethyleneimine coated plates than on polylysine coated plates. J Neurosci Res 32:562–568. https://doi.org/10.1002/jnr.490320411
Li W, Ye Y (2008) Polyubiquitin chains: functions, structures, and mechanisms. Cell Mol Life Sci CMLS 65:2397–2406. https://doi.org/10.1007/s00018-008-8090-6
Lopez-Gonzalez R, Yang D, Pribadi M, Kim TS, Krishnan G, Choi SY, Lee SJ, Coppola G, Gao FB (2019) Partial inhibition of the overactivated Ku80-dependent DNA repair pathway rescues neurodegeneration in C9ORF72-ALS/FTD. Proc Natl Acad Sci USA 116:9628–9633. https://doi.org/10.1073/pnas.1901313116
Love S, Barber R, Wilcock GK (1998) Apoptosis and expression of DNA repair proteins in ischaemic brain injury in man. NeuroReport 20:955–959. https://doi.org/10.1097/00001756-199804200-00001
Martin S, Wilkinson KA, Nishimune A, Henley JM (2007) Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci 8:948–959. https://doi.org/10.1038/nrn2276
Morita Y, Kanei-Ishii C, Nomura T, Ishii S (2005) TRAF7 sequesters c-Myb to the cytoplasm by stimulating its sumoylation. Mol Biol Cell 16:5433–5444. https://doi.org/10.1091/mbc.e05-08-0731
Muller S, Hoege C, Pyrowolakis G, Jentsch S (2001) SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol 2:202–210. https://doi.org/10.1038/35056591
Nishida T, Tanaka H, Yasuda H (2000) A novel mammalian Smt3-specific isopeptidase 1 (SMT3IP1) localized in the nucleolus at interphase. Eur J Biochem/FEBS 267:6423–6427. https://doi.org/10.1046/j.1432-1327.2000.01729.x
Oh Y, Chung KC (2013) UHRF2, a ubiquitin E3 ligase, acts as a small ubiquitin-like modifier E3 ligase for zinc finger protein 131. J Biol Chem 288:9102–9111. https://doi.org/10.1074/jbc.M112.438234
Oh CK, Choi YK, Hwang IY, Ko YU, Chung IK, Yun NR, Oh YJ (2020) RING-finger protein 166 plays a novel pro-apoptotic role in neurotoxin-induced neurodegeneration via ubiquitination of XIAP. Cell Death Dis 11:939. https://doi.org/10.1038/s41419-020-03145-x
Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8:610–616. https://doi.org/10.1016/j.cbpa.2004.09.009
Rodriguez MS, Dargemont C, Hay RT (2001) SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 276:12654–12659. https://doi.org/10.1074/jbc.M009476200
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606. https://doi.org/10.1093/emboj/17.9.2596
Sampson DA, Wang M, Matunis MJ (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276:21664–21669. https://doi.org/10.1074/jbc.M100006200
Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP (2006) The mechanisms of PML-nuclear body formation. Mol Cell 24(5):805. https://doi.org/10.1016/j.molcel.2006.11.010
Socha A, Yang D, Bulsiewicz A, Yaprianto K, Kupculak M, Liang CC, Hadjicharalambous A, Wu R, Gygi SP, Cohn MA (2020) WRNIP1 is recruited to DNA interstrand crosslinks and promotes repair. Cell Rep 32:107850. https://doi.org/10.1016/j.celrep.2020.107850
Song J, Zhang Z, Hu W, Chen Y (2005) Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J Biol Chem 280:40122–40129. https://doi.org/10.1074/jbc.M50705920
Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D (2000) Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102:67–76. https://doi.org/10.1016/s0092-8674(00)00011-8
Terui Y, Saad N, Jia S, McKeon F, Yuan J (2004) Dual role of sumoylation in the nuclear localization and transcriptional activation of NFAT1. J Biol Chem 279:28257–28265. https://doi.org/10.1074/jbc.M403153200
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J 19:94–102. https://doi.org/10.1093/emboj/19.1.94
Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y (2005) Chromosome alignment and segregation regulated by ubiquitination of survivin. Science 310:1499–1504. https://doi.org/10.1126/science.1120160
Weger S, Hammer E, Heilbronn R (2005) Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 579:5007–5012. https://doi.org/10.1016/j.febslet.2005.07.088
Wilkinson KA, Henley JM (2010) Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 428:133–145. https://doi.org/10.1042/BJ20100158
Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J (2009) Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137:133–145. https://doi.org/10.1016/j.cell.2009.01.041
Yang N, Liu S, Qin T, Liu X, Watanabe N, Mayo KH, Li J, Li X (2019) SUMO3 modification by PIAS1 modulates androgen receptor cellular distribution and stability. Cell Commun Signal 17(1):153. https://doi.org/10.1186/s12964-019-0457-9
Yeh ET, Gong L, Kamitani T (2000) Ubiquitin-like proteins: new wines in new bottles. Gene 248:1–14. https://doi.org/10.1016/s0378-1119(00)00139-6
Funding
This work was supported by the Brain Research Program (2017M3C7A10253369 to YJO) and by the Mid-Career Research Program (2019R1A2C1088793 to YJO) through the National Research Foundation (NRF) of Korea funded by Ministry of Science and ICT, and by the Small Grant for Exploratory Research program (2018R1D1A1A02085731 to NY) and by Basic Science Research Program (2021R1I1A1A01047405 to NY) through the NRF funded by the Ministry of Education.
Author information
Authors and Affiliations
Contributions
IYH, NY, and YJO designed the research, and analyzed the data, and wrote manuscript, IYH, CKO, and YKC conducted most of the experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hwang, IY., Oh, CK., Choi, Y.K. et al. RNF166 plays a dual role for Lys63-linked ubiquitination and sumoylation of its target proteins. J Neural Transm 129, 463–475 (2022). https://doi.org/10.1007/s00702-021-02442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02442-9