Abstract
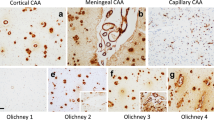
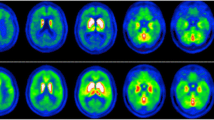
Lewy body dementia (LBD) and Parkinson's disease-dementia (PDD) are two major neurocognitive disorders with Lewy bodies (LB) of unknown etiology. There is considerable clinical and pathological overlap between these two conditions that are clinically distinguished based on the duration of Parkinsonism prior to development of dementia. Their morphology is characterized by a variable combination of LB and Alzheimer's disease (AD) pathologies. Cerebral amyloid angiopathy (CAA), very common in aged persons and particularly in AD, is increasingly recognized for its association with both pathologies and dementia. To investigate neuropathological differences between LB diseases with and without dementia, 110 PDD and 60 LBD cases were compared with 60 Parkinson's disease (PD) cases without dementia (PDND). The major demographic and neuropathological data were assessed retrospectively. PDD patients were significantly older than PDND ones (83.9 vs 77.8 years; p < 0.05); the age of LB patients was in between both groups (mean 80.2 years), while the duration of disease was LBD < PDD < PDND (mean 6.7 vs 12.5 and 14.3 years). LBD patients had higher neuritic Braak stages (mean 5.1 vs 4.5 and 4.0, respectively), LB scores (mean 5.3 vs 4.2 and 4.0, respectively), and Thal amyloid phases (mean 4.1 vs 3.0 and 2.3, respectively) than the two other groups. CAA was more common in LBD than in the PDD and PDND groups (93 vs 50 and 21.7%, respectively). Its severity was significantly greater in LBD than in PDD and PDND (p < 0.01), involving mainly the occipital lobes. Moreover, striatal Aβ deposition highly differentiated LBD brains from PDD. Braak neurofibrillary tangle (NFT) stages, CAA, and less Thal Aβ phases were positively correlated with LB pathology (p < 0.05), which was significantly higher in LBD than in PDD < PDND. Survival analysis showed worse prognosis in LBD than in PDD (and PDND), which was linked to both increased Braak tau stages and more severe CAA. These and other recent studies imply the association of CAA—and both tau and LB pathologies—with cognitive decline and more rapid disease progression that distinguishes LBD from PDD (and PDND).





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Aβ:
-
β-Amyloid
- CAA:
-
Cerebral amyloid angiopathy
- LB:
-
Lewy body
- LBD:
-
Lewy body dementia
- MMSE:
-
Mini Mental State Examination
- PD:
-
Parkinson's disease
- PDD:
-
Parkinson's disease-dementia
- PDND:
-
Parkinson's disease without dementia
- rCBF:
-
Regional cerebral blood flow
- αSyn:
-
α-Synuclein
References
Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, Gage FH (2017) Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 37:1675–1684
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H, (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Alafuzoff I, Thal DR, Arzberger T, Bogdanovic N, Al-Sarraj S, Bodi I, Boluda S, Bugiani O, Duyckaerts C, Gelpi E, Gentleman S, Giaccone G, Graeber M, Hortobagyi T, Höftberger R, Ince P, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Nilsson T, Parchi P, Patsouris E, Pikkarainen M, Revesz T, Rozemuller A, Seilhean D, Schulz-Schaeffer W, Streichenberger N, Wharton SB, Kretzschmar H (2009) Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe Consortium. Acta Neuropathol 117:309–320
Alakbarzade V, French JM, Howlett DR, Attems J, Francis PT, Stratton S, Clark CN, Pereira AC, Hainsworth AH (2021) Cerebral amyloid angiopathy distribution in older people: a cautionary note. Alzheimers Dement (N Y) 7:e12145
American, Association AP (2013) Diagnostic and statistical manual of mental disorders (DSM-5), 5th edn. American Psychiatric Association, Arlington
Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA (2011) Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol 69:320–327
Attems J, Jellinger KA (2004) Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology—a pilot study. Acta Neuropathol 107:83–90
Attems J, Jellinger KA, Lintner F (2005) Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol (Berl) 110:222–231
Attems J, Jellinger K, Thal DR, Van Nostrand W (2011) Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 37:75–93
Ballard C, Ziabreva I, Perry R, Larsen JP, O’Brien J, McKeith I, Perry E, Aarsland D (2006) Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 67:1931–1934
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol (Berl) 94:403–409
Bencs V, Bencze J, Módis VL, Simon V, Kálmán J, Hortobágyi T (2020) Pathological and clinical comparison of Parkinson’s disease dementia and dementia with Lewy bodies] [Article in Hu]. Orv Hetil 161:727–737
Bertrand E, Lewandowska E, Stepien T, Szpak GM, Pasennik E, Modzelewska J (2008) Amyloid angiopathy in idiopathic Parkinson’s disease. Immunohistochemical and ultrastructural study. Folia Neuropathol 46:255–270
Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, Schneider JA (2015) Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85:1930–1936
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Burn DJ, Rowan EN, Minett T, Sanders J, Myint P, Richardson J, Thomas A, Newby J, Reid J, O’Brien JT, McKeith IG (2003) Extrapyramidal features in Parkinson’s disease with and without dementia and dementia with Lewy bodies: a cross-sectional comparative study. Mov Disord 18:884–889
Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127:791–800
Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA (2003) Parkinson’s disease is associated with hippocampal atrophy. Mov Disord 18:784–790
Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, Iannaccone S, Magnani G, Padovani A, Ferini-Strambi L, Perani D (2019) Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res Ther 11:20
Charidimou A, Gang Q, Werring DJ (2012) Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 83:124–137
Chen D, Jiang J, Lu J, Wu P, Zhang H, Zuo C, Shi K (2019) Brain network and abnormal hemispheric asymmetry analyses to explore the marginal differences in glucose metabolic distributions among Alzheimer’s disease, Parkinson’s disease dementia, and Lewy body dementia. Front Neurol 10:369
Chia R, Sabir MS, Bandres-Ciga S, Saez-Atienzar S, Reynolds RH, Gustavsson E, Walton RL, Ahmed S, Viollet C, Ding J, Makarious MB, Diez-Fairen M, Portley MK, Shah Z, Abramzon Y, Hernandez DG, Blauwendraat C, Stone DJ, Eicher J, Parkkinen L, Ansorge O, Clark L, Honig LS, Marder K, Lemstra A, St George-Hyslop P, Londos E, Morgan K, Lashley T, Warner TT, Jaunmuktane Z, Galasko D, Santana I, Tienari PJ, Myllykangas L, Oinas M, Cairns NJ, Morris JC, Halliday GM, Van Deerlin VM, Trojanowski JQ, Grassano M, Calvo A, Mora G, Canosa A, Floris G, Bohannan RC, Brett F, Gan-Or Z, Geiger JT, Moore A, May P, Krüger R, Goldstein DS, Lopez G, Tayebi N, Sidransky E, Norcliffe-Kaufmann L, Palma JA, Kaufmann H, Shakkottai VG, Perkins M, Newell KL, Gasser T, Schulte C, Landi F, Salvi E, Cusi D, Masliah E, Kim RC, Caraway CA, Monuki ES, Brunetti M, Dawson TM, Rosenthal LS, Albert MS, Pletnikova O, Troncoso JC, Flanagan ME, Mao Q, Bigio EH, Rodríguez-Rodríguez E, Infante J, Lage C, González-Aramburu I, Sanchez-Juan P, Ghetti B, Keith J, Black SE, Masellis M, Rogaeva E, Duyckaerts C, Brice A, Lesage S, Xiromerisiou G, Barrett MJ, Tilley BS, Gentleman S, Logroscino G, Serrano GE, Beach TG, McKeith IG, Thomas AJ, Attems J, Morris CM, Palmer L, Love S, Troakes C, Al-Sarraj S, Hodges AK, Aarsland D, Klein G, Kaiser SM, Woltjer R, Pastor P, Bekris LM, Leverenz JB, Besser LM, Kuzma A, Renton AE, Goate A, Bennett DA, Scherzer CR, Morris HR, Ferrari R, Albani D, Pickering-Brown S, Faber K, Kukull WA, Morenas-Rodriguez E, Lleó A, Fortea J, Alcolea D, Clarimon J, Nalls MA, Ferrucci L, Resnick SM, Tanaka T, Foroud TM, Graff-Radford NR, Wszolek ZK, Ferman T, Boeve BF, Hardy JA, Topol EJ, Torkamani A, Singleton AB, Ryten M, Dickson DW, Chiò A, Ross OA, Gibbs JR, Dalgard CL, Traynor BJ, Scholz SW (2021) Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet 53:294–303
Chin KS, Yassi N, Churilov L, Masters CL, Watson R (2020) Prevalence and clinical associations of tau in Lewy body dementias: a systematic review and meta-analysis. Parkinsonism Relat Disord 80:184–193
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM (2010) Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 30:7281–7289
Colom-Cadena M, Grau-Rivera O, Planellas L, Cerquera C, Morenas E, Helgueta S, Muñoz L, Kulisevsky J, Martí MJ, Tolosa E, Clarimon J, Lleó A, Gelpi E (2017) Regional overlap of pathologies in Lewy body disorders. J Neuropathol Exp Neurol 76:216–224
Cong C, Zhang W, Qian X, Qiu W, Ma C (2021) Significant overlap of alpha-synuclein, amyloid-beta, and phospho-tau pathologies in neuropathological diagnosis of Lewy-related pathology. J Alzheimers Dis 80:447–458
Coughlin D, Xie SX, Liang M, Williams A, Peterson C, Weintraub D, McMillan CT, Wolk DA, Akhtar RS, Hurtig HI, Branch Coslett H, Hamilton RH, Siderowf AD, Duda JE, Rascovsky K, Lee EB, Lee VM, Grossman M, Trojanowski JQ, Irwin DJ (2019) Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol 85:259–271
De Reuck J (2019) The impact of cerebral amyloid angiopathy in various neurodegenerative dementia syndromes: a neuropathological study. Neurol Res Int 2019:7247325
Del Ser T, Hachinski V, Merskey H, Munoz DG (2001) Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: effect of coexisting Alzheimer-type lesion load. Alzheimer Dis Assoc Disord 15:31–44
Deramecourt V, Bombois S, Maurage CA, Ghestem A, Drobecq H, Vanmechelen E, Lebert F, Pasquier F, Delacourte A (2006) Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol 65:278–288
Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, Beach TG (2014) Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord 20:525–529
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Någren K, Chaudhury KR, Masters CL, Brooks DJ (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79:1331–1338
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Fereshtehnejad SM, Lökk J, Wimo A, Eriksdotter M (2018) No significant difference in cognitive decline and mortality between Parkinson’s disease dementia and dementia with Lewy bodies: naturalistic longitudinal data from the Swedish Dementia Registry. J Parkinsons Dis 8:553–561
Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, Uitti RJ, Wszolek ZK, Graff-Radford J, Pedraza O, Kantarci K, Boeve BF, Dickson DW (2018) The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimers Dement 14:330–339
Ferman TJ, Aoki N, Boeve BF, Aakre JA, Kantarci K, Graff-Radford J, Parisi JE, Van Gerpen JA, Graff-Radford NR, Uitti RJ, Pedraza O, Murray ME, Wszolek ZK, Reichard RR, Fields JA, Ross OA, Knopman DS, Petersen RC, Dickson DW (2020) Subtypes of dementia with Lewy bodies are associated with α-synuclein and tau distribution. Neurology 95:e155–e165
Ferreira D, Przybelski SA, Lesnick TG, Lemstra AW, Londos E, Blanc F, Nedelska Z, Schwarz CG, Graff-Radford J, Senjem ML, Fields JA, Knopman DS, Savica R, Ferman TJ, Graff-Radford NR, Lowe VJ, Jack CR Jr, Petersen RC, Mollenhauer B, Garcia-Ptacek S, Abdelnour C, Hort J, Bonanni L, Oppedal K, Kramberger MG, Boeve BF, Aarsland D, Westman E, Kantarci K (2020) Beta-amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 95:e3257–e3268
Fritz NE, Kegelmeyer DA, Kloos AD, Linder S, Park A, Kataki M, Adeli A, Agrawal P, Scharre DW, Kostyk SK (2016) Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture 50:1–7
Fujishiro H, Iseki E, Higashi S, Kasanuki K, Murayama N, Togo T, Katsuse O, Uchikado H, Aoki N, Kosaka K, Arai H, Sato K (2010) Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett 486:19–23
Ghebremedhin E, Rosenberger A, Rüb U, Vuksic M, Berhe T, Bickeböller H, de Vos RA, Thal DR, Deller T (2010) Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol 69:442–448
Goetz CG, Emre M, Dubois B (2008) Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 64(Suppl 2):S81-92
Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71:903–910
Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, Vasdev N, Sperling R, Growdon JH, Dickerson BC, Johnson K (2016) Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol 73:1334–1341
Gratwicke J, Oswal A, Akram H, Jahanshahi M, Hariz M, Zrinzo L, Foltynie T, Litvak V (2020) Resting state activity and connectivity of the nucleus basalis of Meynert and globus pallidus in Lewy body dementia and Parkinson’s disease dementia. Neuroimage 221:117184
Gungor I, Sarro L, Graff-Radford J, Zuk SM, Tosakulwong N, Przybelski SA, Lesnick T, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Filippi M, Petersen RC, Jack CR Jr, Kantarci K (2015) Frequency and topography of cerebral microbleeds in dementia with Lewy bodies compared to Alzheimer’s disease. Parkinsonism Relat Disord 21:1101–1104
Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM (2013) Distinct alpha-synuclein strains differentially promote tau inclusions in neurons. Cell 154:103–117
Halliday GM, Song YJ, Harding AJ (2011) Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm (Vienna) 118:713–719
Hansen LA (1997) The Lewy body variant of Alzheimer disease. J Neural Transm Suppl 51:83–93
Hansen D, Ling H, Lashley T, Holton JL, Warner TT (2019) Review: Clinical, neuropathological and genetic features of Lewy body dementias. Neuropathol Appl Neurobiol 45:635–654
Hansen D, Ling H, Lashley T, Foley JA, Strand C, Eid TM, Holton JL, Warner TT (2021) Novel clinicopathological characteristics differentiate dementia with Lewy bodies from Parkinson’s disease dementia. Neuropathol Appl Neurobiol 47:143–156
Heckman MG, Kasanuki K, Diehl NN, Koga S, Soto A, Murray ME, Dickson DW, Ross OA (2017) Parkinson’s disease susceptibility variants and severity of Lewy body pathology. Parkinsonism Relat Disord 44:79–84
Howard E, Irwin DJ, Rascovsky K, Nevler N, Shellikeri S, Tropea TF, Spindler M, Deik A, Chen-Plotkin A, Siderowf A, Dahodwala N, Weintraub D, Shaw LM, Trojanowski JQ, Vaishnavi SN, Wolk DA, Mechanic-Hamilton D, Morley JF, Duda JE, Grossman M, Cousins KA (2021) Cognitive profile and markers of AD-type pathology in patients with Lewy body dementias. Neurology 96:e1855–e1864
Howlett DR, Whitfield D, Johnson M, Attems J, O’Brien JT, Aarsland D, Lai MK, Lee JH, Chen C, Ballard C, Hortobágyi T, Francis PT (2015) Regional multiple pathology scores are associated with cognitive decline in Lewy body dementias. Brain Pathol 25:401–408
Huber M, Beyer L, Prix C, Schönecker S, Palleis C, Rauchmann BS, Morbelli S, Chincarini A, Bruffaerts R, Vandenberghe R, Van Laere K, Kramberger MG, Trost M, Grmek M, Garibotto V, Nicastro N, Frisoni GB, Lemstra AW, van der Zande J, Pilotto A, Padovani A, Garcia-Ptacek S, Savitcheva I, Ochoa-Figueroa MA, Davidsson A, Camacho V, Peira E, Arnaldi D, Bauckneht M, Pardini M, Sambuceti G, Vöglein J, Schnabel J, Unterrainer M, Perneczky R, Pogarell O, Buerger K, Catak C, Bartenstein P, Cumming P, Ewers M, Danek A, Levin J, Aarsland D, Nobili F, Rominger A, Brendel M (2020) Metabolic correlates of dopaminergic loss in dementia with Lewy bodies. Mov Disord 35:595–605
Hughes TA, Ross HF, Mindham RH, Spokes EG (2004) Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 110:118–123
Irwin DJ, Hurtig HI (2018) The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism 8:444
Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72:587–598
Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK, Nelson PT, Jicha GA, Woltjer R, Quinn JF, Kaye J, Leverenz JB, Tsuang D, Longfellow K, Yearout D, Kukull W, Keene CD, Montine TJ, Zabetian CP, Trojanowski JQ (2017) Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 16:55–65
Irwin DJ, Xie SX, Coughlin D, Nevler N, Akhtar RS, McMillan CT, Lee EB, Wolk DA, Weintraub D, Chen-Plotkin A, Duda JE, Spindler M, Siderowf A, Hurtig HI, Shaw LM, Grossman M, Trojanowski JQ (2018) CSF tau and beta-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 90:e1038–e1046
Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K (2003) Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol (Berl) 105:265–270
Jellinger KA (2006) Pathological substrate of dementia in Parkinson’s disease—its relation to DLB and DLBD. Parkinsonism Relat Disord 12:119–120
Jellinger KA (2008) Re: In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 70:407–408
Jellinger KA (2009) Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci 24:114–125
Jellinger KA (2010a) Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegener Dis 7:112–115
Jellinger KA (2010b) The neuropathologic substrate of Parkinson disease dementia. Acta Neuropathol 119:151–153
Jellinger KA (2011) Interaction between alpha-synuclein and other proteins in neurodegenerative disorders. Sci World J 11:1893–1907
Jellinger KA (2012) Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med 16:1166–1183
Jellinger KA (2018) Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna) 125:615–650
Jellinger KA (2020) Striatal dopamine transporter activity may predict ß-amyloid pathology in dementia with Lewy bodies. Neurology 94:557–558
Jellinger KA (2021) Morphological differences between dementia with Lewy bodies and Parkinson's disease-dementia. Letter to the Editor. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12708
Jellinger KA, Attems J (2006a) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol (Berl) 112:253–260
Jellinger KA, Attems J (2006b) Prevalence and impact of cerebrovascular pathology in Alzheimer’s disease and parkinsonism. Acta Neurol Scand 114:38–46
Jellinger KA, Attems J (2008a) Cerebral amyloid angiopathy in Lewy body disease. J Neural Transm (Vienna) 115:473–482
Jellinger KA, Attems J (2008b) Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115:427–436
Jellinger KA, Korczyn AD (2018) Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med 16:34
Jellinger KA, Seppi K, Wenning GK, Poewe W (2002) Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm 109:329–339
Jellinger KA, Lauda F, Attems J (2007a) Sporadic cerebral amyloid angiopathy is not a frequent cause of spontaneous brain hemorrhage. Eur J Neurol 14:923–928
Jellinger KA, Wenning GK, Seppi K (2007b) Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis 4:428–430
Joki H, Higashiyama Y, Nakae Y, Kugimoto C, Doi H, Kimura K, Kishida H, Ueda N, Nakano T, Takahashi T, Koyano S, Takeuchi H, Tanaka F (2018) White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J Neurol Sci 385:99–104
Jokinen P, Scheinin N, Aalto S, Någren K, Savisto N, Parkkola R, Rokka J, Haaparanta M, Röyttä M, Rinne JO (2010) [(11)C]PIB-, [(18)F]FDG-PET and MRI imaging in patients with Parkinson’s disease with and without dementia. Parkinsonism Relat Disord 16:666–670
Kalaitzakis ME, Pearce RK (2009) The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol 118:587–598
Kalaitzakis ME, Walls AJ, Pearce RK, Gentleman SM (2011) Striatal Aß peptide deposition mirrors dementia and differentiates DLB and PDD from other parkinsonian syndromes. Neurobiol Dis 41:377–384
Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, Lesnick TG, Spychalla AJ, Gunter JL, Fields JA, Graff-Radford J, Ferman TJ, Jones DT, Murray ME, Knopman DS, Jack CR Jr, Petersen RC (2017) AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 81:58–67
Kapasi A, Leurgans SE, Arvanitakis Z, Barnes LL, Bennett DA, Schneider JA (2021) Abeta (amyloid beta) and tau tangle pathology modifies the association between small vessel disease and cortical microinfarcts. Stroke 52:1012–1021
Kim SW, Chung SJ, Oh YS, Yoon JH, Sunwoo MK, Hong JY, Kim JS, Lee PH (2015) Cerebral microbleeds in patients with dementia with Lewy bodies and Parkinson disease dementia. AJNR Am J Neuroradiol 36:1642–1647
Lee SH, Cho H, Choi JY, Lee JH, Ryu YH, Lee MS, Lyoo CH (2018) Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Mov Disord 33:262–272
Lemstra AW, de Beer MH, Teunissen CE, Schreuder C, Scheltens P, van der Flier WM, Sikkes SA (2017) Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 88:113–118
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, Bradshaw J, Merory J, Woodward M, Hopwood M, Rowe CC (2009) The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med 50:1638–1645
Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K, Yamada M, McCarron M, Minett T, Matthews F, Greenberg S, Mann D, Kehoe PG (2014) Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am J Neurodegener Dis 3:19–32
Maetzler W, Reimold M, Liepelt I, Solbach C, Leyhe T, Schweitzer K, Eschweiler GW, Mittelbronn M, Gaenslen A, Uebele M, Reischl G, Gasser T, Machulla HJ, Bares R, Berg D (2008) [11C]PIB binding in Parkinson’s disease dementia. Neuroimage 39:1027–1033
Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ (2020) Braak stage, cerebral amyloid angiopathy, and cognitive decline in early Alzheimer’s disease. J Alzheimers Dis 74:189–197
Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R (2006) Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res 31:1153–1162
Martini A, Weis L, Schifano R, Pistonesi F, Fiorenzato E, Antonini A, Biundo R (2020) Differences in cognitive profiles between Lewy body and Parkinson’s disease dementia. J Neural Transm (Vienna) 127:323–330
Matar E, Shine JM, Halliday GM, Lewis SJG (2020) Cognitive fluctuations in Lewy body dementia: towards a pathophysiological framework. Brain 143:31–46
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89:88–100
Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K (2005) Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64:224–229
Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, Gunter JL, Senjem ML, Vemuri P, Smith GE, Geda YE, Graff-Radford J, Knopman DS, Petersen RC, Parisi JE, Dickson DW, Jack CR Jr, Kantarci K (2015) Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 36:452–461
Nichols JB, Malek-Ahmadi M, Tariot PN, Serrano GE, Sue LI, Beach TG (2021) Vascular lesions, APOE epsilon4, and tau pathology in Alzheimer disease. J Neuropathol Exp Neurol 80:240–246
Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H (2008) Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol 210:409–420
Oliveira FPM, Walker Z, Walker RWH, Attems J, Castanheira JC, Silva Ã, Oliveira C, Vaz S, Silva M, Costa DC (2021) (123)I-FP-CIT SPECT in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’s disease: a new quantitative analysis of autopsy confirmed cases. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2020-324606
Oswal A, Gratwicke J, Akram H, Jahanshahi M, Zaborszky L, Brown P, Hariz M, Zrinzo L, Foltynie T, Litvak V (2021) Cortical connectivity of the nucleus basalis of Meynert in Parkinson’s disease and Lewy body dementias. Brain 144:781–788
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, Attems J, McKeith I (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14:5
Petrova M, Mehrabian-Spasova S, Aarsland D, Raycheva M, Traykov L (2015) Clinical and neuropsychological differences between mild Parkinson’s disease dementia and dementia with Lewy bodies. Dement Geriatr Cogn Dis Extra 5:212–220
Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, Dawson TM, Marsh L, Troncoso JC (2005) Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging 26:1183–1192
Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J (2009) Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol 118:115–130
Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV (2014) Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol 71:878–883
Ryman SG, Yutsis M, Tian L, Henderson VW, Montine TJ, Salmon DP, Galasko D, Poston KL (2021) Cognition at each stage of Lewy body disease with co-occurring Alzheimer’s disease pathology. J Alzheimers Dis 80:1243–1256
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S (2003) Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol 62:644–654
Salkovic-Petrisic M, Osmanovic-Barilar J, Brückner MK, Hoyer S, Arendt T, Riederer P (2011) Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm (Vienna) 118:765–772
Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, Ota T, Asahina M, Fukushi K, Kuwabara S, Hattori T, Suhara T, Irie T (2009) Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73:273–278
Shirvan J, Clement N, Ye R, Katz S, Schultz A, Johnson KA, Gomez-Isla T, Frosch M, Growdon JH, Gomperts SN (2019) Neuropathologic correlates of amyloid and dopamine transporter imaging in Lewy body disease. Neurology 93:e476–e484
Sierra M, Gelpi E, Martí MJ, Compta Y (2016) Lewy- and Alzheimer-type pathologies in midbrain and cerebellum across the Lewy body disorders spectrum. Neuropathol Appl Neurobiol 42:451–462
Smirnov DS, Galasko D, Edland SD, Filoteo JV, Hansen LA, Salmon DP (2020) Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology 94:e2076–e2087
Smith EE, Schneider JA, Wardlaw JM, Greenberg SM (2012) Cerebral microinfarcts: the invisible lesions. Lancet Neurol 11:272–282
Smith C, Malek N, Grosset K, Cullen B, Gentleman S, Grosset DG (2019) Neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J Neurol Neurosurg Psychiatry 90:1234–1243
Spires-Jones TL, Attems J, Thal DR (2017) Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 134:187–205
Spotorno N, Coughlin DG, Olm CA, Wolk D, Vaishnavi SN, Shaw LM, Dahodwala N, Morley JF, Duda JE, Deik AF, Spindler MA, Chen-Plotkin A, Lee EB, Trojanowski JQ, McMillan CT, Weintraub D, Grossman M, Irwin DJ (2020) Tau pathology associates with in vivo cortical thinning in Lewy body disorders. Ann Clin Transl Neurol 7:2342–2355
Swirski M, Miners JS, de Silva R, Lashley T, Ling H, Holton J, Revesz T, Love S (2014) Evaluating the relationship between amyloid-beta and alpha-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res Ther 6:77
Thal DR, Rub U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H (2000) Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–748
Thomas A, Ballard C, Kenny RA, O’Brien J, Oakley A, Kalaria R (2005) Correlation of entorhinal amyloid with memory in Alzheimer’s and vascular but not Lewy body dementia. Dement Geriatr Cogn Disord 19:57–60
Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, Sedor S, Waits K, Boluda S, Grossman M, Van Deerlin VM, Lee EB, Arnold SE, Duda JE, Hurtig H, Lee VM, Adler CH, Beach TG, Trojanowski JQ (2016) Pathological alpha-synuclein distribution in subjects with coincident Alzheimer’s and Lewy body pathology. Acta Neuropathol 131:393–409
Tsai HH, Tsai LK, Lo YL, Lin CH (2021) Amyloid related cerebral microbleed and plasma Abeta40 are associated with cognitive decline in Parkinson’s disease. Sci Rep 11:7115
Tsuboi Y, Dickson DW (2005) Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord 11(Suppl 1):S47-51
van der Zande JJ, Steenwijk MD, Ten Kate M, Wattjes MP, Scheltens P, Lemstra AW (2018) Gray matter atrophy in dementia with Lewy bodies with and without concomitant Alzheimer’s disease pathology. Neurobiol Aging 71:171–178
Walker Z, Possin KL, Boeve BF, Aarsland D (2015) Lewy body dementias. Lancet 386:1683–1697
Walker L, Stefanis L, Attems J (2019) Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—current issues and future directions. J Neurochem 150:467–474
Weil RS, Lashley TL, Bras J, Schrag AE, Schott JM (2017) Current concepts and controversies in the pathogenesis of Parkinson’s disease dementia and Dementia with Lewy Bodies. F1000Res 6:1604
Wolters EE, van de Beek M, Ossenkoppele R, Golla SSV, Verfaillie SCJ, Coomans EM, Timmers T, Visser D, Tuncel H, Barkhof F, Boellaard R, Windhorst AD, van der Flier WM, Scheltens P, Lemstra AW, van Berckel BNM (2020) Tau PET and relative cerebral blood flow in dementia with Lewy bodies: a PET study. Neuroimage Clin 28:102504
Wu E, Lipton RB, Dickson DW (1992) Amyloid angiopathy in diffuse Lewy body disease. Neurology 42:2131–2135
Yoo HS, Lee S, Chung SJ, Lee YH, Lee PH, Sohn YH, Yun M, Ye BS (2020a) Dopaminergic depletion, beta-amyloid burden, and cognition in Lewy body disease. Ann Neurol 87:739–750
Yoo HS, Lee S, Chung SJ, Lee YH, Ye BS, Sohn YH, Yun M, Lee PH (2020b) Clinical and striatal dopamine transporter predictors of beta-amyloid in dementia with Lewy bodies. Neurology 94:e1344–e1352
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl, PhD, for secretarial and editorial work.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Significance of cerebral amyloid angiopathy and other co-morbidities in Lewy body diseases. J Neural Transm 128, 687–699 (2021). https://doi.org/10.1007/s00702-021-02345-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02345-9