Abstract
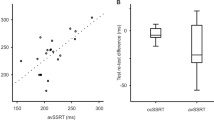
Instrumental measurement of response assets and movement behaviour gained importance as addition to rating procedures to determine the efficacy of therapeutic interventions in patients with Parkinson’s disease. Objectives were to determine the response to standardised 100 mg levodopa application with repeat performance of complex and simple instrumental tests in relation to scored motor behaviour in 53 previously treated patients. Levodopa improved rating scores of motor impairment, execution of complicated movement patterns and complex reaction time. Computed improvements in these instrumental test results correlated with each other. Execution of the simple reaction time paradigm and of plain movement sequences did not ameliorate after levodopa. The changes of these simple test results were not associated to each other. These different response patterns result from the higher cognitive demand of dopamine sensitive association areas of the prefrontal cortex and mesolimbic system for the complex test execution in contrast to the simple task performance.






Similar content being viewed by others
References
Akram H, Wu C, Hyam J, Foltynie T, Limousin P, De VE, Yousry T, Jahanshahi M, Hariz M, Behrens T, Ashburner J, Zrinzo L (2017) l-Dopa responsiveness is associated with distinctive connectivity patterns in advanced Parkinson's disease. Mov Disord 32:874–883
Birn RM, Bandettini PA, Cox RW, Shaker R (1999) Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7:106–114
Calabresi P, Picconi B, Parnetti L, Di FM (2006) A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol 5:974–983
Chini B, Leonzino M, Braida D, Sala M (2014) Learning about oxytocin: pharmacologic and behavioral issues. Biol Psychiatry 76:360–366
Cools R, Frobose M, Aarts E, Hofmans L (2019) Dopamine and the motivation of cognitive control. Handb Clin Neurol 163:123–143
Espay AJ, Morgante F, Lang AE, Chen R (2009) Impairments of speed and amplitude of movement in Parkinson's disease: a pilot study. Mov Disord 24(7):1001–1008
Fabbri M, Coelho M, Guedes LC, Chendo I, Sousa C, Rosa MM, Abreu D, Costa N, Godinho C, Antonini A, Ferreira JJ (2017) Response of non-motor symptoms to levodopa in late-stage Parkinson's disease: results of a levodopa challenge test. Parkinsonism Relat Disord 39:37–43
Fahn S, Elton RL (1987) Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M (eds) Recent devolpments in Parkinson’s disease. Macmillon Healthcare Information, Florham Park, pp 153–163
Garber CE, Friedman JH (2003) Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology 60:1119–1124
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Goetz CG, Stebbins GT, Wolff D, Deleeuw W, Bronte-Stewart H, Elble R, Hallett M, Nutt J, Ramig L, Sanger T, Wu AD, Kraus PH, Blasucci LM, Shamim EA, Sethi KD, Spielman J, Kubota K, Grove AS, Dishman E, Taylor CB (2008) Testing objective measures of motor impairment in early Parkinson's disease: feasibility study of an at-home testing device. Mov Disord 24:549–554
Goren JL, Friedman JH (1998) Yawning as an aura for an L-dopa-induced "on" in Parkinson's disease. Neurology 50:823
Gratton C, Yousef S, Aarts E, Wallace DL, D'Esposito M, Silver MA (2017) Cholinergic, but not dopaminergic or noradrenergic, enhancement sharpens visual spatial perception in humans. J Neurosci 37:4405–4415
Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM (2004) Motor Sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cognit Neurosci 16:621–636
Hensler JG, Artigas F, Bortolozzi A, Daws LC, De DP, Milan L, Navailles S, Koek W (2013) Catecholamine/serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol 68:167–197
Korf J, Loopuijt LD (1988) Synaptic and non-synaptic striatal dopamine D2 receptors: possible implications in normal and pathological behaviour. Acta Morphol Neerl Scand 26:177–190
Lakie M, Mutch WJ (1989) Finger tremor in Parkinson's disease. J Neurol Neurosurg Psychiatry 52:392–394
Lalonde R, Botez-Marquard T (1997) The neurobiological basis of movement initiation. Rev Neurosci 8:35–54
Leal PC, Bispo JMM, Engelberth RCGJ, Silva KD, Meurer YR, Ribeiro AM, Silva RH, Marchioro M, Santos JR (2019) Serotonergic dysfunction in a model of parkinsonism induced by reserpine. J Chem Neuroanat 96:73–78
Loiodice S, Wing YH, Rion B, Meot B, Montagne P, Denibaud AS, Viel R, La Drieu RC (2019) Implication of nigral dopaminergic lesion and repeated L-dopa exposure in neuropsychiatric symptoms of Parkinson's disease. Behav Brain Res 360:120–127
Martinez-Fernandez R, Schmitt E, Martinez-Martin P, Krack P (2016) The hidden sister of motor fluctuations in Parkinson's disease: a review on nonmotor fluctuations. Mov Disord 31:1080–1094
Mueller K, Jech R, Ballarini T, Holiga S, Ruzicka F, Piecha FA, Moller HE, Vymazal J, Ruzicka E, Schroeter ML (2019) Modulatory effects of levodopa on cerebellar connectivity in Parkinson's disease. Cerebellum 18:212–224
Müller T (2014) Tolcapone addition improves Parkinson's disease associated nonmotor symptoms. Ther Adv Neurol Disord 7:77–82
Müller T, Benz S (2002) Quantification of the dopaminergic response in Parkinson's disease. Parkinsonism Relat Disord 8:181–186
Müller T, Benz S, Przuntek H (2000a) Choice reaction time after levodopa challenge in Parkinsonian patients. J Neurol Sci 181:98–103
Müller T, Schäfer S, Kuhn W, Przuntek H (2000b) Correlation between tapping and inserting of pegs in Parkinson's disease. Can J Neurol Sci 27:311–315
Müller T, Benz S, Börnke C (2001) Delay of simple reaction time after levodopa intake. Clin Neurophysiol 112:2133–2137
Müller T, Benz S, Przuntek H (2002) Tapping and peg insertion after levodopa intake in treated and de novo parkinsonian patients. Can J Neurol Sci 29:73–77
Müller T, Benz S, Bornke C, Przuntek H (2004) Differential response in choice reaction time following apomorphine based on prior dopaminergic treatment. Acta Neurol Scand 109:348–354
Müller T, Öhm G, Eilert K, Möhr K, Rotter S, Haas T, Küchler M, Lütge S, Marg M, Rothe H (2017) Benefit on motor and non-motor behavior in a specialized unit for Parkinson's disease. J Neural Transm (Vienna) 124:715–720
Navailles S, Di GG, De DP (2014) Predicting dopaminergic effects of L-DOPA in the treatment for Parkinson's disease. CNS Neurosci Ther 20:699–701
Nguyen A, Roth N, Ghassemi NH, Hannink J, Seel T, Klucken J, Gassner H, Eskofier BM (2019) Development and clinical validation of inertial sensor-based gait-clustering methods in Parkinson's disease. J Neuroeng Rehabil 16:77
Nieoullon A, Coquerel A (2003) Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol 16(Suppl 2):S3–S9
Pal PK, Lee CS, Samii A, Schulzer M, Stoessl AJ, Mak EK, Wudel J, Dobko T, Tsui JK (2001) Alternating two finger tapping with contralateral activation is an objective measure of clinical severity in Parkinson's disease and correlates with PET. Parkinsonism Relat Disord 7:305–309
Przuntek H, Müller T, Riederer P (2004) Diagnostic staging of Parkinson's disease: conceptual aspects. J Neural Transm (Vienna) 111:201–216
Purba JS, Hofman MA, Swaab DF (1994) Decreased number of oxytocin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus in Parkinson's disease. Neurology 44:84–89
Pursiainen V, Lyytinen J, Pekkonen E (2012) Effect of duodenal levodopa infusion on blood pressure and sweating. Acta Neurol Scand 126:e20–e24
Taylor Tavares AL, Jefferis GS, Koop M, Hill BC, Hastie T, Heit G, Bronte-Stewart HM (2005) Quantitative measurements of alternating finger tapping in Parkinson's disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord 20:1286–1298
Teive HAG, Munhoz RP, Camargo CHF, Walusinski O (2018) Yawning in neurology: a review. Arq Neuropsiquiatr 76:473–480
Trujillo P, van Wouwe NC, Lin YC, Stark AJ, Petersen KJ, Kang H, Zald DH, Donahue MJ, Claassen DO (2019) Dopamine effects on frontal cortical blood flow and motor inhibition in Parkinson's disease. Cortex 115:99–111
Vizcarra JA, Sanchez-Ferro A, Maetzler W, Marsili L, Zavala L, Lang AE, Martinez-Martin P, Mestre TA, Reilmann R, Hausdorff JM, Dorsey ER, Paul SS, Dexheimer JW, Wissel BD, Fuller RLM, Bonato P, Tan AH, Bloem BR, Kopil C, Daeschler M, Bataille L, Kleiner G, Cedarbaum JM, Klucken J, Merola A, Goetz CG, Stebbins GT, Espay AJ (2019) The Parkinson's disease e-diary: developing a clinical and research tool for the digital age. Mov Disord 34:676–681
Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA (2002) Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 59:408–413
Acknowledgement
We thank Tanja Steiner, Bettina Marchewitz, Gudrun Edler, Ute Claussnitzer and Christine Stamm for technical assistance. We thank the participating PD patients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Müller, T., Harati, A. Different response to instrumental tests in relation to cognitive demand after dopaminergic stimulation in previously treated patients with Parkinson’s disease. J Neural Transm 127, 265–272 (2020). https://doi.org/10.1007/s00702-020-02148-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02148-4