Abstract
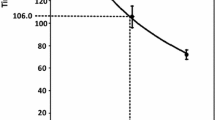
Botulinum toxin (BT) consists of botulinum neurotoxin and complexing proteins (CPs). CPs might provide mechanical protection for botulinum neurotoxin. As incobotulinumtoxinA (INCO, Xeomin®) does not contain CPs, we wanted to compare its mechanical stability to that of onabotulinumtoxinA (ONA, Botox®) containing CPs. For this, ONA and INCO were reconstituted without mechanical stress (NS) and with mechanical stress (WS) generated by a recently introduced stress test. Potencies were then measured by the paralysis times (PTs) in the mouse diaphragm assay. ONA-PT was 75.8 ± 10.3 min (n = 6) under NS and 116.7 ± 29.8 min (n = 6) under WS (two-tailed t test, p = 0.002). Mechanical stress increased the ONA-PT by 35.0% on the Growth Percentage Index. INCO-PT was 66.0 ± 7.0 min for NS and 76.0 ± 1.0 min for WS (t test, p = 0.129). Mechanical stress increased the INCO-PT by 13.2% on the Growth Percentage Index. Our data show that mechanical stress inactivates a CP-containing BT drug, but not a CP-free BT drug. We conclude that CPs do not provide protection against mechanical stress, supporting the view that CPs are not necessary for therapeutic purposes.
Similar content being viewed by others
References
Aoki KR, Ranoux D, Wissel J (2006) Using translational medicine to understand clinical differences between botulinum toxin formulations. Eur J Neurol 13(Suppl 4):10–19
Buelbring F (1946) Observations on the isolated phrenic nerve diaphragm preparation in the rat. Br J Pharmacol 1:38–61
Chen F, Kuziemko GM, Stevens RC (1998) Biophysical characterization of the stability of the 150-kilodalton botulinum toxin, the nontoxic component, and the 900-kilodalton botulinum toxin complex species. Infect Immun 66:2420–2425
De Almeida AT, de Boulle K (2007) Diffusion characteristics of botulinum neurotoxin products and their clinical significance in cosmetic applications. J Cosmet Laser Ther 9(Suppl 1):17–22
Dressler D, Bigalke H (2016) Reconstituting botulinum toxin drugs: shaking, stirring or what? J Neural Transm 123:523–525
Dressler D, Bigalke H (2017) Long-term stability of reconstituted incobotulinumtoxinA: how can we reduce costs of botulinum toxin therapy? J Neural Transm 124:1223–1225
Dressler D, Foster K (2018) Pharmacology of Botulinum Toxins. In: Dressler D, Altenmüller E, Krauss JK (Eds) Treatment of Dystonia. Cambridge University Press, Cambridge.
Eisele KH, Fink K, Vey M, Taylor HV (2011) Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon 57:555–565
Frevert J, Dressler D (2010) Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 4:325–332
Fujinaga Y (2006) Transport of bacterial toxins into target cells: pathways followed by cholera toxin and botulinum progenitor toxin. J Biochem. 140:155–160
Goschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H (1997) Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies: therapeutic consequences. Exp Neurol 147:96–102
Jin Y, Takegahara Y, Sugawara Y, Matsumura T, Fujinaga Y (2009) Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins–differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155:35–45
Johnson EA, Bradshaw M (2001) Clostridium botulinum and its neurotoxins: a metabolic and cellular perspective. Toxicon 39:1703–1722
Ohishi I, Sugii S, Sakaguchi G (1977) Oral toxicities of Clostridium botulinum toxins in response to molecular size. Infect Immun 16:107–109
Pickett A, Dodd S, Rzany B (2008) Confusion about diffusion and the art of misinterpreting data when comparing different botulinum toxins used in aesthetic applications. J Cosmet Laser Ther 10:181–183
Rossetto O, Pirazzini M, Montecucco C (2014) Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol 12:535–549
Shome D, Nair AG, Kapoor R, Jain V (2010) Botulinum toxin A: is it really that fragile a molecule? Dermatol Surg 36(Suppl 4):2106–2110
Tang-Liu DD, Aoki KR, Dolly JO, de Paiva A, Houchen TL, Chasseaud LF, Webber C (2003) Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon 42:461–469
Acknowledgements
Statistical help of Dr. Leijla Paracka is much appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DD received honoraria for services provided to Allergan, Ipsen, Merz, Lanzhou Institute of Biological Products, Medy-Tox, Revance, Desitin, Syntaxin, Abbvie, Medtronic, St Jude, Boston Scientific, Almirall, Bayer, Sun, Teva, UCB, IAB-Interdisciplinary Working Group for Movement Disorders. He is a shareholder of Allergan and holds patents on botulinum toxin and botulinum toxin therapy. LP has nothing to declare. FAS received honoraria for services provided to Abbvie, Almirall, Allergan, Asklepios, Bayer, Desitin, Dynamed, Hempel GesundheitsPartner, Ipsen, Johnson & Johnson, Licher, Meda, Medtronic, Medizinische Hochschule Hannover, Merz, Orion, ParkinsonNet, PTZ Nawrath, Sensomotorik & Rehabilitation Hellmuth & Thiel, Sintetica, Sporlastic, Sun, Teva, Tricumed, TRS Med and UCB. HB has nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Dressler, D., Pan, L., Adib Saberi, F. et al. Do complexing proteins provide mechanical protection for botulinum neurotoxins?. J Neural Transm 126, 1047–1050 (2019). https://doi.org/10.1007/s00702-019-02023-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02023-x