Abstract
In patients with Parkinson’s disease (PD), abnormal activations of nociceptive brain areas and lowered pain thresholds were reported, probably reflecting a central modification of pain processing. The aim of this study was to investigate the possible correlation between the striatal and extrastriatal dopaminergic system and pain threshold in PD patients. We included 25 PD patients with various intensities of central pain (visual analog scale). Subjective pain threshold (thermotest) and a motor examination (UPDRS III) were performed. Patients underwent SPECT imaging with [123I]-FP-CIT. We analyzed the correlation between [123I]-FP-CIT binding and subjective pain threshold, using a simple linear regression model for striatal uptake and a voxel-based approach for extrastriatal uptake. The covariables were age, sex, duration of PD, and UPDRS motor score. A pain matrix mask was also used to identify clusters in relation with pain matrix. Striatal analysis revealed that [123I]-FP-CIT binding was negatively correlated with age (p = 0.02), duration of PD (p = 0.0002) and UPDRS motor score (p = 0.006), but no correlation with pain threshold was observed. The extrastriatal analysis showed a positive correlation between [123I]-FP-CIT binding and subjective heat pain threshold for the left posterior cingulate cortex (PCC) (p < 0.001) and negative correlations for the right secondary visual cortex (p < 0.001) and left insula (p < 0.001). When applying the pain matrix mask, correlations remained significant only in the left PCC and the left insula. We suggest that pain perception abnormalities in PD are not directly related to striatal dopaminergic dysfunction. Painful sensations may be related to extrastriatal monoaminergic systems.
Similar content being viewed by others
Introduction
Non-motor features are now well described in Parkinson’s disease (PD) patients. One of the most disabling and frequent non-motor feature is pain. Several studies have shown that 70–80% of patients experience painful sensations (Beiske et al. 2009; Defazio et al. 2008; Negre-Pages et al. 2008) and that the prevalence of pain in PD patients is higher than that in the general population (Brefel-Courbon et al. 2009). It has been suggested that there is a central modification of pain processing in PD patients. Pain perception abnormalities, such as lowered pain thresholds and a lower pain tolerance than observed in healthy subjects, and a modification of nociceptive laser-evoked potential amplitude have been reported in PD patients with and without pain (Brefel-Courbon et al. 2005; Djaldetti et al. 2004; Gerdelat-Mas et al. 2007; Lim et al. 2008; Mylius et al. 2009; Schestatsky et al. 2007; Tinazzi et al. 2008; Zambito Marsala et al. 2010). In addition, H215O positron emission tomography (PET) studies performed during experimental painful stimulation, in PD patients both with and without pain, found evidence of abnormal hyperactivation in nociceptive brain areas underlying discriminative, affective-motivational and cognitive aspects of pain (Brefel-Courbon et al. 2005, 2013).
The administration of levodopa was found to reduce pain sensitivity, to raise pain thresholds and to decrease cerebral hyperactivation (Brefel-Courbon et al. 2005; Gerdelat-Mas et al. 2007; Slaoui et al. 2007). This antinociceptive effect of levodopa may be related to a striatal and/or extrastriatal dopaminergic effect. Indeed, there is neuroanatomical, electrophysiological, and pharmacological evidence to suggest that basal ganglia (including the nigrostriatal dopaminergic pathway) plays a role in nociceptive mechanisms (Chudler and Dong 1995) and that dopaminergic receptors are present in brain areas involved in pain processing, such as the anterior cingulate cortex, posterior cingulate cortex, insula, and prefrontal cortex (Hurd et al. 2001).
We investigated the possible involvement of striatal and/or extrastriatal dopaminergic system dysfunction in the pain perception abnormalities of PD patients, by investigating the correlation between striatal and extrastriatal [123I]-FP-CIT uptake in single-photon emission computed tomography (SPECT) and the subjective pain threshold.
Methods
Patients
We included patients with a clinical diagnosis of PD according to the UKPDSBB criteria (Gibb and Lees 1988; Hughes et al. 1992). Patients were asked whether they suffered from painful sensations, and to indicate the average intensity of pain over the last week on a 100-mm visual analog scale (VAS). Patients indicating an absence of pain were included in the study as pain-free patients, whereas those indicating that they experienced pain were questioned to determine whether they had central neuropathic pain caused by PD.
The causal link between PD and pain was assessed with a five-question clinical questionnaire developed at Toulouse Hospital and based on a consensus between movement disorder and pain experts. Pain was defined as related to PD if the patient reported at least three of the following five features (Dellapina et al. 2012):
-
Pain occurring at the onset of PD or influenced by motor condition
-
Pain influenced by dopaminergic drugs
-
Pain located in the half of the body most severely affected by PD
-
Pain not related to other evident etiologies (rheumatic, traumatic or orthopedic disorders)
-
Link between pain and PD established by the patient
Central pain was identified by interviewing patients with the DN4 questionnaire, and checking for an absence of radicular systematization (Wasner and Deuschl 2012). If patients suffered from mixed pain (central neuropathic and nociceptive), the intensity of their central pain had to be greater than that of the nociceptive pain for the pain to be classified as central pain. PD patients with nociceptive pain only were excluded. We therefore established two groups of patients: pain-free patients and patients with central pain caused by PD. We focused on patients with central pain because this kind of pain is thought to be a direct consequence of changes in central pain processing (Wasner and Deuschl 2012).
We evaluated the motor state of the patients with the Unified Parkinson’s Disease Rating Scale (UPDRS) motor scale (part III). All had rigidity and akinesia scores of more than 2 points on UPDRS part III for the most affected side. The exclusion criteria were: Raynaud disease, severe depression [Depression score on the Hospital Anxiety and Depression Scale (HADS) ≥ 15], cognitive impairment (MMS < 26) and acute or chronic pain with another etiology, such as rheumatism or orthopedic disease. All PD patients were taking dopaminergic drugs (levodopa and/or dopamine agonists). All patients underwent first the clinical pain perception evaluations and then the SPECT scanning.
All these assessments were performed after the withdrawal of dopaminergic treatment for at least 12 h and of analgesic treatment and extended-release dopamine agonists for at least 24 h to allow an assessment of the subjective pain threshold without drug interference.
Ethics committee approval and written informed consent were obtained. This trial is registered with ClinicalTrials.gov, number NCT00940914.
Pain assessment
Subjective heat pain threshold was assessed by Peltier-based contact temperature stimulation with a 12 × 25 mm contact thermode (MSA Thermotest, Somedic AB, Sweden) (Fruhstorfer et al. 1976). Heat pain threshold was measured on the thenar on the most affected side of the body, by the method of levels (Defrin et al. 2004), which does not take the reaction time of PD patients into account (this reaction time is often altered in OFF medication conditions). The temperature of the thermode (initially at 30 °C) was increased in 3 °C steps. At the end of the stimulation, which lasted 30 s, the patients were asked whether or not they felt pain. After the first report of pain, the temperature of the next stimulus was decreased by 1.5 °C. If the patient reported pain in response to this stimulus, the temperature was decreased by a further 0.75 °C, whereas, if the patient reported no pain in response to this stimulus, the temperature was increased by 0.75 °C. For each subsequent stimulus, the difference in temperature between successive stimuli was halved, down to 0.2 °C.
[123I]-FP-CIT SPECT
All patients underwent brain SPECT with 123I-ioflupane-FP-CIT tracer (DaTSCAN, Amersham, GE Healthcare). As FP-CIT was radiolabeled with [123I], potassium perchlorate (200 mg) was administered to each patient 15 min before the injection of [123I]-FP-CIT and 8 h after SPECT scan, to protect against iodine capture by the thyroid. Each patient received an intravenous injection of 123 MBq of 123I-ioflupane-FP-CIT (range, 98–214 MBq). Brain SPECT acquisition was performed 3 h after the radiotracer injection and lasted 30 min. SPECT images were obtained with a Pickers IRIX (Philips Healthcare, Eindhoven, Netherlands) gamma camera equipped with three fixed rotating detectors, with low-energy, high-resolution (LEHR) parallel collimators. We used a 360-degree acquisition around the head, with 120 projections (40 per detector) separated by 3°. Images were reconstructed from photopeak counts (159 ± 16 keV) with filtered back projection algorithms and 3D post-filtering (Butterworth filter, order 4, cutoff frequency 0.35). The spatial resolution of the system was 9 mm in full-width at half maximum (FWHM) mode, with a 128 × 128 matrix.
Image analysis
Images were first converted from DICOM to Neuro-Imaging Informatics Technology Initiative (NIfTI) format, with Statistical Parametric Mapping (SPM 8) software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab R2016b (Mathworks Inc., Sherborn, MA, USA).
Parametric images of binding potential (BP) were computed from the reconstructed [123I]-FP-CIT volumes with Brainvisa software (http://brainvisa.info). A spherical volume of interest drawn within the occipital cortex, was chosen to evaluate non-specific activity due to its low level of monoaminergic transporters (Garcia-Larrea 2012) BP was calculated for each voxel, as follows:
The resulting parametric volumes were then spatially normalized into Montreal Neurological Institute (MNI) space with SPM8 software, using a customized [123I]-FP-CIT template (Kas et al. 2007).
Statistical analysis
This was a pilot study investigating the possible relationship between quantitative variables, such as subjective pain threshold, and [123I]-FP-CIT uptake. Assuming a negative correlation between these two variables, with a regression coefficient of − 0.6, we would need to include at least 20 patients to obtain a power of 90%. Clinical values are expressed as mean values ± standard deviation (SD). We checked that the variables were normally distributed, in Shapiro–Wilk tests. We then used Spearman’s rank correlation analysis to investigate the relationship between subjective pain threshold and VAS score. We also investigated the relationship between HADS subscores (depression and anxiety) and VAS score on one hand and the subjective pain thresholds on the other using Spearman’s rank correlation analyses.
Striatal analysis
This analysis was performed in the native space (i.e., before spatial normalization), with SUSI (Specific Uptake Size Index) software (Tossici-Bolt et al. 2006). Definition of the image for the quantification of striatal uptake was automated. It included summed transaxial slices of about 44 mm in thickness and was centered on the strongest striatal signal. Striatal uptake was quantified by a ROI approach. Striatal ROIs had an a priori shape including the whole striatum of each side of the body (Fig. 1). Simple linear regression models were used to evaluate the correlation between striatal [123I]-FP-CIT uptake in the most affected side of the body (i.e., the striatum contralateral to the side used for subjective pain threshold determinations) and subjective pain threshold, age, PD duration and UPDRS motor score. Bonferroni-Holm correction was applied. Results were considered to be significant if p < 0.05. Statistical analyses of the results obtained for the striatum were performed with STATISTICA 9.1 software (Statsoft, 2010).
Voxel-based extrastriatal analysis
The SPECT data were smoothed with a Gaussian kernel of 8 × 8 × 8 mm at FWHM. A whole-brain voxel-based analysis was then performed after spatial normalization, with no prior assumptions. Whole-brain correlations were performed by computing voxel-based statistics with SPM8 (version 6313). A general linear model was used to perform appropriate voxel-by-voxel univariate statistical tests, using several covariates as regressors (multiple regression analysis). We focused on correlations between subjective heat pain threshold and whole-brain dopamine transporter (DaT) levels, with age, sex, UPDRS part III and PD duration as confounding variables. Positive and negative correlations were calculated. This process generated t statistics for each voxel and established a statistical parametric map SPM{t}. Proportional scaling was not used for global normalization in SPM options. Instead, we used binding potential index (BPI). The SPM{t} map was thresholded on a default probability p < 0.001 (uncorrected) and with a minimum spatial extent voxel cluster size of k = 20. Regions that were significant were then identified, with their coordinates, with the MRIcron tool (http://www.nitrc.org/projects/mricron). These areas were displayed as the maximum intensity projected in an axial slice for a spatially normalized single MRI T1 subject. Only clusters surviving to cluster extend and corrections are reported. In addition, a pain matrix mask was generated with WFU Pickatlas toolbox (Hsieh et al. 1995) by creating the union of Brodmann areas 1, 2, 3, 4, 5, 6, and 25 as well as the thalamus, anterior cingulate cortex (ACC) and the insula from the AAL atlas (Hughes et al. 1992) (all dilated to 3 mm in 3D) according to the “Pain Matrix” described recently (Hurd et al. 2001). This mask was then used to identify clusters in relation with pain matrix.
Voxel-based striatal analysis
In addition to ROI-based striatal analysis using SUSI, we performed a voxel-based striatal analysis. The preprocessing was identical to the whole-brain extrastriatal analysis. Voxel-based striatal correlations were performed by computing voxel-based statistics with SPM8 using an inclusive mask of striatum. In this part, inclusive mask was used to performed voxel-based statistical analysis limited on striatum. The striatum mask was made using pickatlas toolbox (http://fmri.wfubmc.edu/software/pickatlas) implemented in Matlab R2016b (Mathworks Inc., Sherborn, MA, USA). The same way as voxel-based extrastriatal analysis, a general linear model was used to perform appropriate voxel-by-voxel univariate statistical tests, using subjective heat pain threshold and whole-brain dopamine transporter (DaT) levels as regressor, with age, sex, UPDRS part III and PD duration as confounding variables. Positive and negative correlations were calculated.
Results
In total, 25 of the PD patients seen in the Neurology Department of Toulouse University were included in this study.
The baseline characteristics and clinical data for these patients are summarized in Table 1. The clinical characteristics of the central pain caused by PD are summarized in Table 2.
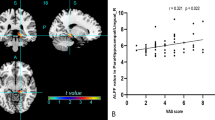
We found a significant negative correlation between VAS score and subjective heat pain threshold (adjusted R2 = 0.20; p = 0.01).
Neither VAS score nor the subjective pain threshold were significantly correlated with the HADS depression score (p = 0.98 for each correlation analysis). Similarly, neither VAS score nor the subjective pain threshold were significantly correlated with the HADS anxiety score (p = 0.11 and p = 0.96, respectively).
ROI-based striatal uptake
Striatal [123I]-FP-CIT uptake was negatively correlated with age (adjusted R2 = 0.18; p = 0.04), PD duration (adjusted R2 = 0.42; p = 0.001) and UPDRS motor score (adjusted R2 = 0.25; p = 0.02). No significant correlation was found between striatal [123I]-FP-CIT uptake and subjective heat pain threshold (adjusted R2 = − 0.04; p = 0.95).
Voxel-based extrastriatal uptake
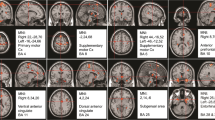
The extrastriatal analysis showed a significant positive correlation between [123I]-FP-CIT binding and subjective heat pain threshold for the left posterior cingulate cortex (p < 0.001) and significant negative correlations for the right secondary visual cortex (p < 0.001) and left insula (p < 0.001) (Fig. 2; Table 3). Using the pain matrix mask, significant correlations were only found in the left posterior cingulate cortex and the left insula.
Voxel-based striatal uptake
The voxel-based striatal analysis showed no significant correlation at p < 0.001, uncorrected for multiple comparisons. The striatal uptake seems to be not linked with the subjective pain threshold.
Discussion
The principal findings of this study were as follows: (1) there was no significant correlation between striatal [123I]-FP-CIT uptake and subjective heat pain threshold; (2) there were significant correlations between [123I]-FP-CIT uptake in extrastriatal structures and subjective heat pain threshold.
As expected, we found a significant relationship between pain intensity, as assessed on a 100 mmVAS, and subjective heat pain threshold in the 25 PD patients (Djaldetti et al. 2004; Kumru et al. 2012). We also found significant negative correlations between striatal [123I]-FP-CIT uptake and PD duration, age, and UPDRS motor score. These findings have been reported in previous studies (Benamer et al. 2000; Volkow et al. 1996), confirming the accuracy of the method of quantification.
By contrast, there was no significant relationship between striatal [123I]-FP-CIT uptake and subjective heat pain threshold in ROI-based analysis. The results were identical for voxel-based striatal analysis. This lack of significance may be due to the low power of the study (small number of patients). However, with the same number of patients, we found significant correlations between [123I]-FP-CIT uptake and other clinical parameters (PD duration, age, UPDRS motor score), suggesting that this result is probably valid, and not due to noise or statistical nuisances. Thus, this lack of correlation suggests that nigrostriatal dopaminergic neuronal loss is not directly related to pain perception abnormalities in PD.
Although nigrostriatal denervation is the pathological hallmark of PD, it is now well known that pathological processes are not restricted to the striatal dopaminergic system. Instead, they also affect extrastriatal dopaminergic (dopaminergic receptors are widely distributed in the brain, particularly in regions involved in pain processing, such as the anterior cingulate cortex, posterior cingulate cortex, insula, and prefrontal cortex) and non-dopaminergic systems (such as serotonin, noradrenergic) (Hurd et al. 2001).
Concerning the extrastriatal uptake analysis, we found a significant positive correlation between subjective heat pain threshold and [123I]-FP-CIT uptake in the left posterior cingulate cortex (BA31). Thus, lower subjective pain thresholds were associated with lower [123I]-FP-CIT uptake in the posterior cingulate cortex. This area is involved in pain perception processing (Becerra et al. 1999; Bromm 2001; Nielsen et al. 2005), including the “emotional-aversive” component of pain in particular (Bromm 2001). We also found a significant negative correlation in the left posterior insula and in right secondary visual cortex (located outside the volume of interest chosen for the assessment of non-specific activity) but this last region disappeared when applying the pain matrix mask. Thus, lower subjective pain thresholds were associated with higher levels of [123I]-FP-CIT uptake in these regions in PD patients. The insula has been shown to receive a high density of dopamine fibers arising from mesencephalic areas, and dopamine has been implicated in the modulation of pain in the insular cortex (Coffeen et al. 2010; Xie et al. 2009). The insula is involved in the lateral discriminative pain pathways associated with sensory discriminative aspects of pain, and the posterior insula is even considered to be the “third somatosensory region” (S3), because of its relationships with the thalamus, limbic, and multisensory region (contribution to thermosensory, nociceptive, and C-fiber tactile input) (Coghill et al. 1999; Garcia-Larrea 2012; Peyron et al. 2000). Thus, PD patients with lower subjective heat pain thresholds displayed lower levels of [123I]-FP-CIT uptake in the left posterior cingulate cortex, which is part of the medial pain system, and higher levels of uptake in the left insula, which is part of the lateral pain system. These findings suggest a possible imbalance between the medial and lateral pain pathways in PD patients and a predominance of dopaminergic denervation in the medial affective nociceptive pathway related to central neuropathic pain. These findings are consistent with our previous results (Brefel-Courbon et al. 2013) showing that PD patients with central pain display the preferential recruitment of medial pain systems. Moreover, other studies have suggested that chronic neuropathic pain may result from changes in areas associated with the affective/emotional dimension of pain (Hsieh et al. 1995; Moisset and Bouhassira 2007). We suggest the possible involvement of this imbalance between pain pathways in the occurrence of central pain in PD. However [123I]-FP-CIT binds non-selectively to both dopaminergic and serotonin transporters. While [123I]-FP-CIT binding in the striatum mainly reflects presynaptic dopamine transporter availability, binding in the extrastriatal area could reflect serotonin transporter availability. Serotonin transporter is located with high density in the midbrain, thalamus, putamen, and caudate and intermediate binding is found in anterior cingulate and frontal cortex (Schestatsky et al. 2007). According to our results, high regional serotonin transporter binding in the anterior insula was associated with low tonic pain ratings in healthy subjects (Slaoui et al. 2007). Moreover, positive correlations between heat pain ratings and 5HT2A receptors binding in posterior cingulate cortex were also reported (Stern 2014). Hence, we could hypothesize that extrastriatal monoaminergic systems (dopaminergic or serotoninergic) could modulate pain perception in PD patients.
This study had several methodological strengths and limitations. We used the most robust methods to explore both the striatal and extrastriatal dopaminergic systems. We chose a validated ROI and a voxel-based approach for the investigation of [123I]-FP-CIT uptake in the striatum. The SUSI program provides an accurate, sensitive, and reproducible quantification of the background-subtracted striatal uptake ratio for FP-CIT in brain images (Tossici-Bolt et al. 2006). For extrastriatal quantification, rather than selecting pain-related regions of interest, we used a whole-brain voxel-based analysis method, not subject to prior assumptions. Given the exploratory nature of this study, we used a threshold of p < 0.001 (uncorrected) for this work, rather than a more conservative correction [such as family-wise error (FWE) or false discovery rate (FDR)]. Voxel-based regression analysis has inherent limitations in terms of spatial normalization and image registration due to its spatial resolution. We are aware that SPECT imaging with [123I]-FP-CIT is not the most suitable method for explorations of cortex activation. However, we used an innovative method for spatial normalization, which improved the accuracy of the whole-brain coregistration (Kas et al. 2007), then we used same voxel-based analysis in striatum and in extrastriatal, to compare the results of correlations. Finally, the patients suffering of severe depression were excluded based on the clinical diagnosis of the investigator and the HADS depression score and most of the patients did not suffer from any depression symptoms (HADS depression score ≤ 7). In addition, our results showed no significant correlation between depression and anxiety scores and pain parameters (VAS score and pain threshold) suggesting that these psychological parameters do not strongly influence the results.
In conclusion, dopamine is known to play a role in modulating pain perception in supraspinal regions. These results suggest that nigrostriatal dopaminergic systems are not directly involved in the pain perception abnormalities of PD patients and that extrastriatal monoaminergic systems may be responsible, through the dysregulation of pain pathways in PD. Future studies should investigate more precisely other monoamine systems, such as the noradrenergic and serotoninergic systems, which may be involved in the pathophysiology of pain in PD and should evaluate the correlation between pain threshold and [123I]-FP-CIT uptake in other groups of patients without PD but suffering from central neuropathic pain.
References
Becerra LR et al (1999) Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 41:1044–1057
Beiske AG, Loge JH, Ronningen A, Svensson E (2009) Pain in Parkinson’s disease prevalence characteristics. Pain 141:173–177
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15:692–698
Brefel-Courbon C et al (2005) Effect of levodopa on pain threshold in Parkinson’s disease: a clinical and positron emission tomography study. Mov Disord 20:1557–1563
Brefel-Courbon C et al (2009) Comparison of chronic analgesic drugs prevalence in Parkinson’s disease other chronic diseases the general population. Pain 141:14–18
Brefel-Courbon C, Ory-Magne F, Thalamas C, Payoux P, Rascol O (2013) Nociceptive brain activation in patients with neuropathic pain related to Parkinson’s disease. Parkinsonism Relat Disord 19:548–552
Bromm B (2001) Brain images of pain. News Physiol Sci 16:244–249
Chudler EH, Dong WK (1995) The role of the basal ganglia in nociception and pain. Pain 60:3–38
Coffeen U, Ortega-Legaspi JM, de Gortari P, Simon-Arceo K, Jaimes O, Amaya MI, Pellicer F (2010) Inflammatory nociception diminishes dopamine release and increases dopamine D2 receptor mRNA in the rat’s insular cortex. Mol Pain 6:75
Coghill RC, Sang CN, Maisog JM, Iadarola MJ (1999) Pain intensity processing within the human brain: a bilateral distributed mechanism. J Neurophysiol 82:1934–1943
Defazio G et al (2008) Pain as a nonmotor symptom of Parkinson disease: evidence from a case–control study. Arch Neurol 65:1191–1194. https://doi.org/10.1001/archneurol.2008.2
Defrin R, Pick CG, Peretz C, Carmeli E (2004) A quantitative somatosensory testing of pain threshold in individuals with mental retardation. Pain 108:58–66
Dellapina E et al (2012) Effect of subthalamic deep brain stimulation on pain in Parkinson‘s disease. Pain 153:2267–2273
Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D (2004) Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 62:2171–2175
Fruhstorfer H, Lindblom U, Schmidt WC (1976) Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry 39:1071–1075
Garcia-Larrea L (2012) The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin 42:299–313
Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C (2007) Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 78:1140–1142
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M (1995) Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain 63:225–236
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Hurd YL, Suzuki M, Sedvall GC (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat 22:127–137
Kas A et al (2007) Validation of a standardized normalization template for statistical parametric mapping analysis of 123I-FP-CIT images. J Nucl Med 48:1459–1467
Kumru H, Soler D, Vidal J, Tormos JM, Pascual-Leone A, Valls-Sole J (2012) Evoked potentials and quantitative thermal testing in spinal cord injury patients with chronic neuropathic pain. Clin Neurophysiol 123:598–604
Lim SY, Farrell MJ, Gibson SJ, Helme RD, Lang AE, Evans AH (2008) Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson’s disease? Mov Disord 23:1689–1695
Moisset X, Bouhassira D (2007) Brain imaging of neuropathic pain. Neuroimage 37(Suppl 1):S80–S88
Mylius V et al (2009) Pain sensitivity and descending inhibition of pain in Parkinson’s disease. J Neurol Neurosurg Psychiatry 80:24–28
Negre-Pages L, Regragui W, Bouhassira D, Grandjean H, Rascol O (2008) Chronic pain in Parkinson’s disease: the cross-sectional French DoPaMiP survey. Mov Disord 23:1361–1369
Nielsen FA, Balslev D, Hansen LK (2005) Mining the posterior cingulate: segregation between memory pain components. Neuroimage 27:520–532
Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30:263–288
Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, Chaves ML (2007) Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 69:2162–2169
Slaoui T, Mas-Gerdelat A, Ory-Magne F, Rascol O, Brefel-Courbon C (2007) [Levodopa modifies pain thresholds in Parkinson’s disease patients]. Rev Neurol (Paris) 163:66–71 (MDOI-RN-01-2007-163-1-0035-3787-101019-200604714 [pii])
Stern AF (2014) The hospital anxiety and depression scale. Occup Med 64:393–394 https://doi.org/10.1093/occmed/kqu024
Tinazzi M et al (2008) Abnormal processing of the nociceptive input in Parkinson’s disease: a study with CO2 laser evoked potentials. Pain 136:117–124
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS (2006) Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging 33:1491–1499. https://doi.org/10.1007/s00259-006-0155-x
Volkow ND et al (1996) Dopamine transporters decrease with age. J Nucl Med 37:554–559
Wasner G, Deuschl G (2012) Pains in Parkinson disease–many syndromes under one umbrella. Nat Rev Neurol 8:284–294
Xie YF, Huo FQ, Tang JS (2009) Cerebral cortex modulation of pain. Acta Pharmacol Sin 30:31–41
Zambito Marsala S et al (2010) Spontaneous pain, pain threshold, and pain tolerance in Parkinson’s disease. J Neurol 258:627–633
Acknowledgements
This work was supported by a Toulouse University Hospital grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dellapina, E., Pellaprat, J., Adel, D. et al. Dopaminergic denervation using [123I]-FPCIT and pain in Parkinson’s disease: a correlation study. J Neural Transm 126, 279–287 (2019). https://doi.org/10.1007/s00702-019-01974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01974-5