Abstract
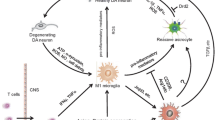
Neuroinflammation is a main component of Parkinson’s disease (PD) neuropathology, where unremitting reactive microglia and microglia-secreted soluble molecules such as cytokines, contribute to the neurodegenerative process as part of an aberrant immune reaction. Besides, pro-inflammatory cytokines, predominantly TNF-α, play an important neuromodulatory role in the healthy and diseased brain, being involved in neurotransmitter metabolism, synaptic scaling and brain plasticity. Recent preclinical studies have evidenced an exacerbated neuroinflammatory reaction in the striatum of parkinsonian rats that developed dyskinetic responses following l-DOPA administration. These findings prompted investigation of non-neuronal mechanisms of l-DOPA-induced dyskinesia (LID) involving glial cells and glial-secreted soluble molecules. Hence, besides the classical mechanisms of LID that include abnormal corticostriatal neurotransmission and maladaptive changes in striatal medium spiny neurons (MSNs), here we review studies supporting a role of striatal neuroinflammation in the development of LID, with a focus on microglia and the pro-inflammatory cytokine TNF-α. Moreover, we discuss several mechanisms that have been involved in the development of LID, which are directly or indirectly under the control of TNF-α, and might be abnormally affected by its chronic overproduction and release by microglia in PD. It is proposed that TNF-α may contribute to the altered neuronal responses occurring in LID by targeting receptor trafficking and function in MSNs, but also dopamine synthesis in preserved dopaminergic terminals and serotonin metabolism in serotonergic neurons. Therapeutic approaches specifically targeting glial-secreted cytokines may represent a novel target for preventing or treating LID.


Similar content being viewed by others
References
Antonini A, Fung VSC, Boyd JT, Slevin JT, Hall C, Chatamra K, Eaton S, Benesh JA (2016) Effect of levodopa–carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov Disord 31:530–537
Ba M, Kong M, Yang H, Ma G, Lu G, Chen S, Liu Z (2006) Changes in subcellular distribution and phosphorylation of GluR1 in lesioned striatum of 6-hydroxydopamine-lesioned and l-dopa-treated rats. Neurochem Res 31:1337–1347
Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A (2005) Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol 57(6):804–812
Balosso S, Ravizza T, Pierucci M, Calcagno E, Invernizzi R, Di Giovanni G, Esposito E, Vezzani A (2009) Molecular and functional interactions between tumor necrosis factor-alpha receptors and the glutamatergic system in the mouse hippocampus: implications for seizure susceptibility. Neuroscience 161(1):293–300
Barcia C, Hunot S, Guillemin GJ, Pitossi F (2011) Inflammation and Parkinson’s disease. Parkinsons Dis 2011:729054
Barnum CJ, Eskow KL, Dupre K, Blandino P Jr, Deak T, Bishop C (2008) Exogenous corticosterone reduces l-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta. Neuroscience 156:30–41
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNF alpha. Science 295:2282–2285
Bishop C, George JA, Buchta W, Goldenberg AA, Mohamed M, Dickinson SO, Eissa S, Eskow Jaunarajs KL (2012) Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats. Eur J Neurosci 36(6):2839–2848
Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC (1994) Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neuroscience 172(1–2):151–154
Bortolanza M, Cavalcanti-Kiwiatkoski R, Padovan-Neto FE, da Silva CA, Mitkovski M, Raisman-Vozari R, Del-Bel E (2015a) Glial activation is associated with l-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinson’s disease. Neurobiol Dis 73:377–387
Bortolanza M, Padovan-Neto FE, Cavalcanti-Kiwiatkoski R, Dos Santos-Pereira M, Mitkovski M, Raisman-Vozari R, Del-Bel E (2015b) Are cyclooxygenase-2 and nitric oxide involved in the dyskinesia of Parkinson’s disease induced by l-DOPA? Philos Trans R Soc Lond B Biol Sci 370:1672
Boulanger LM (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64(1):93–109. https://doi.org/10.1016/j.neuron.2009.09.001
Boulanger LM, Huh GS, Shatz CJ (2001) Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol 11(5):568–578
Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B (2010) Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol 9(11):1106–1117
Carta M, Bezard E (2011) Contribution of pre-synaptic mechanisms to l-DOPA-induced dyskinesia. Neuroscience 198:245–251
Carta M, Carlsson T, Kirik D, Bjorklund A (2007) Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130(7):1819–1833
Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E (2011) Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 194:250–261
Carta AR, Mulas G, Bortolanza M, Duarte T, Pillai E, Fisone G, Vozari RR, Del-Bel E (2017) DOPA-induceddyskinesia and neuroinflammation: do microglia and astrocytes play a role? Eur J Neurosci 45(1):73–91
Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D’Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29(11):3442–3452
Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, Sandkühler J (2015) Selective activation of microglia facilitates synaptic strength. J Neurosci 35:4552–4570
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9(1):46–56
Datla KP, Blunt SB, Dexter DT (2001) Chronic l-DOPA administration is not toxic to the remaining dopaminergic nigrostriatal neurons, but instead may promote their functional recovery, in rats with partial 6-OHDA or FeCl(3) nigrostriatal lesions. Mov Disord 16:424–434
Del-Bel E, Padovan-Neto FE, Bortolanza M, Tumas V, Aguiar AS Jr, Raisman-Vozari R, Prediger RD (2015) Nitric oxide, a new player in l-DOPA-induced dyskinesia? Front Biosci 7:168–192
Diaz NL, Waters CH (2009) Current strategies in the treatment of Parkinson’s disease and a personalized approach to management. Expert Rev Neurother 9:1781–1789
Dos-Santos-Pereira M, da Silva CA, Guimarães FS, Del-Bel E (2016) Co-administration of cannabidiol and capsazepine reduces l-DOPA-induced dyskinesia in mice: possible mechanism of action. Neurobiol Dis 94:179–195
Dziewczapolski G, Murer MG, Agid Y, Gershanik O, RaismanVozari R (1997) Absence of neurotoxicity of chronic l-DOPA in 6-hydroxydopamine-lesioned rats. NeuroReport 8:975–979
Fahn S, Parkinson Study Group (2005) Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol 252:IV37–IV42
Fasano S, Bezard E, D’Antoni A, Francardo V, Indrigo M, Qin L, Dovero S, Cerovic M, Cenci MA, Brambilla R (2010) Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with l-dopa-induced dyskinesia. Proc Natl Acad Sci USA 107:21824–21829
Fernández-Calle R, Vicente-Rodríguez M, Gramage E, de la Torre-Ortiz C, Pérez-García C, Ramos MP, Herradón G (2017) Endogenous pleiotrophin and midkine regulate LPS-induced glial responses. Neurosci Lett 662:213–218
Ferrario JE, Delfino MA, Stefano AV, Zbarsky V, Douhou A, Murer MG, Raisman-Vozari R, Gershanik OS (2003) Effects of orally administered levodopa on mesencephalic dopaminergic neurons undergoing a degenerative process. Neurosci Res 47:431–436
Ferrario JE, Taravini IR, Mourlevat S, Stefano A, Delfino MA, Raisman-Vozari R, Murer MG, Ruberg M et al (2004) Differential gene expression induced by chronic levodopa treatment in the striatum of rats with lesions of the nigrostriatal system. J Neurochem 90:1348–1358
Feyder M, Bonito-Oliva A, Fisone G (2011) l-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmission. Front Behav Neurosci 5:71
Fidalgo C, Ko WK, Tronci E, Li Q, Stancampiano R, Chuan Q, Bezard E, Carta M (2015) Effect of serotonin transporter blockade on l-DOPA-induced dyskinesia in animal models of Parkinson’s disease. Neuroscience 298:389–396
Gardoni F, Picconi B, Ghiglieri V, Polli F, Bagetta V, Bernardi G, Cattabeni F, Di Luca M, Calabresi P (2006) A critical interaction between NR2B and MAGUK in l-DOPA induced dyskinesia. J Neurosci 26:2914–2922
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Gerhard A (2016) TSPO imaging in parkinsonian disorders. Clin Transl Imaging 4:183–190
Gil SJ, Park CH, Lee JE, Minn YK, Koh HC (2011) Positive association between striatal serotonin level and abnormal involuntary movements in chronic l-DOPA-treated hemiparkinsonian rats. Brain Res Bull 84(2):151–156
Gu F, Chauhan V, Chauhan A (2015) Glutathione redox imbalance in brain disorders. Curr Opin Clin Nutr Metab Care 18:89–95
Guillemin GJ, Smythe G, Takikawa O, Brew BJ (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49(1):15–23
Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, Suter T, Volterra A (2015) Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell 163(7):1730–1741. https://doi.org/10.1016/j.cell.2015.11.023
Havelund JF, Andersen AD, Binzer M, Blaabjerg M, Heegaard NHH, Stenager E, Faergeman NJ, Gramsbergen JB (2017) Changes in kynurenine pathway metabolism in Parkinson patients with l-DOPA-induced dyskinesia. J Neurochem 142(5):756–766
Idriss HT, Naismith JH (2000) TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech 50(3):184–195
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Joers V, Tansey MG, Mulas G, Carta AR (2017) Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol 155:57–75
Julien C, Berthiaume L, Hadj-Tahar A, Rajput AH, Bedard PJ, Di Paolo T, Julien P, Calon F (2006) Postmortem brain fatty acid pro- file of levodopa-treated Parkinson disease patients and parkinsonian monkeys. Neurochem Int 48:404–414
Kim JH, Lee HW, Hwang J, Kim J, Leem MJ, Han HS, Lee WH, Suk K (2012) Microglia-inhibiting activity of Parkinson’s disease drug amantadine. Neurobiol Aging 33(9):2145–2159
Knott C, Stern G, Kingsbury A, Welcher AA, Wilkin GP (2002) Elevated glial brain-derived neurotrophic factor in Parkinson’s diseased nigra. Parkinsonism Relat Disord 8(5):329–341
Koshimori Y, Ko JH, Mizrahi R, Rusjan P, Mabrouk R, Jacobs MF, Christopher L, Hamani C, Lang AE, Wilson AA, Houle S, Strafella AP (2015) Imaging striatal microglial activation in patients with Parkinson’s disease. PLoS One 10(9):e0138721
Lee JY, Yang HJ, Kim JM, Jeon BS (2013) Novel GCH-1 mutations and unusual long-lasting dyskinesias in Korean families with dopa-responsive dystonia. Parkinsonism Relat Disord 19(12):1156–1159
Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC (2004) TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol 1(3):263–273
Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D (2014) An adaptive role of TNFa in the regulation of striatal synapses. J Neurosci 34:6146–6155
Lipski J, Nistico R, Berretta N, Guatteo E, Bernardi G, Mercuri NB (2011) l-DOPA: a scapegoat for accelerated neurodegeneration in Parkinson’s disease? Prog Neurobiol 94:389–407
López González I, Garcia-Esparcia P, Llorens F, Ferrer I (2016) Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt–Jakob and tauopathies. Int J Mol Sci 17(2):206
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15(1):120–132
Lundblad M, Usiello A, Carta M, Håkansson K, Fisone G, Cenci MA (2005) Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol 194(1):66–75
Marin I, Kipnis J (2013) Learning and memory… and the immune system. Learn Mem 20(10):601–606
Martinez AA, Morgese MG, Pisanu A, Macheda T, Paquette MA, Seillier A, Cassano T, Carta AR et al (2015) Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA- lesioned rats. Neurobiol Dis 74:295–304
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm 5:45
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord 23:474–483
Mena MA, Casarejos MJ, Carazo A, Paıno CL, Garcıa de Yebenes J (1997) Glia protect fetal midbrain dopamine neurons in culture from l-DOPA toxicity through multiple mechanisms. J Neural Transm 104:317–328
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30(4):297–306
Mogi M, Kondo T, Mizuno Y, Nagatsu T (2007) p53 protein, interferon gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett 414:94–97
Montgomery SL, Bowers WJ (2012) Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol 7(1):42–59
Mulas G, Espa E, Fenu S, Spiga S, Cossu G, Pillai E, Carboni E, Simbula G, Jadžić D, Angius F, Spolittu S, Batetta B, Lecca D, Giuffrida A, Carta AR (2016) Differential induction of dyskinesia and neuroinflammation by pulsatile versus continuous l-DOPA delivery in the 6-OHDA model of Parkinson’s disease. Exp Neurol 286:83–92
Olanow CW (2015) Levodopa: effect on cell death and the natural history of Parkinson’s disease. Mov Disord 30:37–44
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levo- dopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Olmos G, Lladó J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat Inflamm 2014:861231. https://doi.org/10.1155/2014/861231
Ossola B, Schendzielorz N, Chen SH, Bird GS, Tuominen RK, Männistö PT, Hong JS (2011) Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia. Neuropharmacology 61(4):574–582
Ouchi Y, Yagi S, Yokokura M, Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S200–S204
Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA induced dyskinesia. Nat Neurosci 6:501–506
Pisanu A, Lecca D, Mulas Wardas J, Simbula G, Spiga S, Carta AR (2014) Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis 71:280–291
Pocock JM, Kettenmann H (2007) Neurotransmitter receptors on microglia. Trends Neurosci 30:527–535
Pons R, Syrengelas D, Youroukos S, Orfanou I, Dinopoulos A, Cormand B, Ormazabal A, Garzía-Cazorla A, Serrano M, Artuch R (2013) Levodopa-induced dyskinesias in tyrosine hydroxylase deficiency. Mov Disord 28(8):1058–1063
Pribiag H, Stellwagen D (2014) Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78:13–22
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 342:1484–1491
Rossi S, Furlan R, De Chiara V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, Battistini L, Martino G, Centonze D (2012a) Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol 71(1):76–83
Rossi S, Studer V, Motta C, De Chiara V, Barbieri F, Bernardi G, Centonze D (2012b) Inflammation inhibits GABA transmission in multiple sclerosis. Mult Scler 18:1633–1635
Ryan BJ, Lourenço-Venda LL, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R (2014) α-Synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med 67:58–68
Sakai N, Kaufman S, Milstien S (1995) Parallel induction of nitric oxide and tetrahydrobiopterin synthesis by cytokines in rat glial cells. J Neurochem 65:895–902
Salter MW, Beggs S (2014) Sublime microglia: expanding roles for the guardians of the CNS. Cell 158:15–24
Santello M, Volterra A (2012) TNFα in synaptic function: switching gears. Trends Neurosci 35(10):638–647
Santello M, Bezzi P, Volterra A (2011) TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69(5):988–1001. https://doi.org/10.1016/j.neuron.2011.02.003
Santini E, Valjent Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA-induced dyskinesia. J Neurosci 27:6995–7005
Sawada M, Imamura K, Nagatsu T (2006) Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm Suppl 70:373–381
Schwarcz R, Pellicciari R (2002) Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303(1):1–10
Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, Zucca FA (2014) Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem 129:898–915
Silver K, Desormaux A, Freeman LC, Lillich JD (2012) Expression of pleiotrophin, an important regulator of cell migration, is inhibited in intestinal epithelial cells by treatment with non-steroidal anti-inflammatory drugs. Growth Factors 30(4):258–266
Silverdale MA, Kobylecki C, Hallett PJ, Li Q, Dunah AW, Ravenscroft P, Bezard E, Brotchie JM (2010) Synaptic recruitment of AMPA glutamate receptor subunits in levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate. Synapse 64:177–180
Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440:1054–1059
Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. Neuroscience 25:3219–3228
Sulzer D, Zecca L (2000) Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res 1:181–195
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281(30):21362–21368
Tassin J, Dürr A, Bonnet AM, Gil R, Vidailhet M, Lücking CB, Goas JY, Durif F, Abada M, Echenne B, Motte J, Lagueny A, Lacomblez L, Jedynak P, Bartholomé B, Agid Y, Brice A (2000) Levodopa-responsive dystonia. GTP cyclohydrolase I or parkin mutations? Brain 123(Pt 6):1112–1121
Tremblay ME, Lowery RL, Majewska AK (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8:e1000527
Tronci E, Lisci C, Stancampiano R, Fidalgo C, Collu M, Devoto P, Carta M (2013) 5-Hydroxy-tryptophan for the treatment of l-DOPA-induced dyskinesia in the rat Parkinson’s disease model. Neurobiol Dis 60:108–114
Tronci E, Napolitano F, Muñoz A, Fidalgo C, Rossi F, Björklund A, Usiello A, Carta M (2017) BDNF over-expression induces striatal serotonin fiber sprouting and increases the susceptibility to l-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Exp Neurol 297:73–81
Tronel C, Largeau B, Ribeiro MS, Guilloteau D, Dupont AC, Arlicot N (2017) Molecular targets for PET imaging of activated microglia: the current situation and future expectations. Int J Mol Sci 18(12):802
Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135(3):422–435
Vann LR, Payne SG, Edsall LC, Twitty S, Spiegel S, Milstien S (2002) Involvement of sphingosine kinase in TNF-α-stimulated tetrahydrobiopterin biosynthesis in C6 glioma Ccells. J Biol Chem 277(15):12649–12656
Vezzani A, Viviani B (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96(Pt A):70–82. https://doi.org/10.1016/j.neuropharm.2014.10.027
Vitkovic L, Bockaert J, Jacque C (2000) “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem 74(2):457–471
Wolf SA, Boddeke HW, Kettenmann H (2017) Microglia in physiology and disease. Annu Rev Physiol 79:619–643. https://doi.org/10.1146/annurev-physiol-022516-034406
Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015) Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol 36(10):605–613. https://doi.org/10.1016/j.it.2015.08.008
Zhu CB, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31(10):2121–2131
Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of our wonderful and unforgettable colleague, Dr. Sandro Fenu, who recently passed away.
Rights and permissions
About this article
Cite this article
Pisanu, A., Boi, L., Mulas, G. et al. Neuroinflammation in l-DOPA-induced dyskinesia: beyond the immune function. J Neural Transm 125, 1287–1297 (2018). https://doi.org/10.1007/s00702-018-1874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1874-4