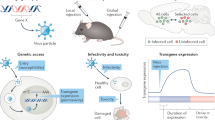
Abstract
To understand the mechanisms underlying higher brain functions, we need to analyze the roles of specific neuronal pathways or cell types forming the complex neural networks. In the neuroscience field, the transgenic approach has provided a useful gene engineering tool for experimental studies of neural functions. The conventional transgenic technique requires the appropriate promoter regions that drive a neuronal type-specific gene expression, but the promoter sequences specifically functioning in each neuronal type are limited. Previously, we developed novel types of lentiviral vectors showing high efficiency of retrograde gene transfer in the central nervous system, termed highly efficient retrograde gene transfer (HiRet) vector and neuron-specific retrograde gene transfer (NeuRet) vector. The HiRet and NeuRet vectors enable genetical manipulation of specific neural pathways in diverse model animals in combination with conditional cell targeting, synaptic transmission silencing, and gene expression systems. These newly developed vectors provide powerful experimental strategies to investigate, more precisely, the machineries exerting various neural functions. In this review, we give an outline of the HiRet and NeuRet vectors and describe recent representative applications of these viral vectors for studies on neural circuits.




Similar content being viewed by others
References
Alstermark B, Isa T, Ohki Y, Saito Y (1999) Disynaptic pyramidal excitation in forelimb motoneurons mediated via C3–C4 propriospinal neurons in the Macaca fuscata. J Neurophysiol 82:3580–3585
Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K (1998) Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3:177–188
Aschauer DF, Kreuz S, Rumpel S (2013) Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8:e76310
Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmellet P, Mazarakis ND (2004) VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429:413–417
Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J (2006) 1-Methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res 83:233–242
Baumgartner BJ, Shine HD (1998) Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol 153:102–112
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015) Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162:622–634
Bru T, Salinas S, Kremer EJ (2010) An update on canine adenovirus type 2 and its vectors. Viruses 2:2134–2153
Burk JA, Mair RG (1998) Thalamic amnesia reconsidered: excitotoxic lesions of the intralaminar nuclei, but not the mediodorsal nucleus, disrupt place delayed matching-to sample performance in rats (Rattus norvegicus). Behav Neurosci 112:54–67
Callaway EM (2008) Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol 18:617–623
Callaway EM, Luo L (2015) Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci 35:8979–8985
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570–6577
Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H (2009) Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75
Federici T, Kutner R, Zhang X-Y, Kuroda H, Tordo N, Boulis NM, Reiser J (2009) Comparative analysis of HIV-1-based lentiviral vectors bearing lyssavirus glycoproteins for neuronal gene transfer. Genet Vaccines Ther 7:1
Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K (2014) Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods 11:763–772
Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89:5547–5551
Govek EE, Newey SE, Van Aelst L (2005) The role of the Rho GTPases in neuronal development. Genes Dev 19:1–49
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15:638–644
Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM (2016) Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron 90:1312–1324
Gu H, Zou YR, Rajewsky K (1993) Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73:1155–1164
Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K (1994) Deletion of a DNA polymerase b gene segment in T cells using cell type-specific gene targeting. Science 265:103–106
Hikosaka O, Nakamura K, Sakai K, Nakahara H (2002) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12:217–222
Hirano M, Kato S, Kobayashi K, Okada T, Yaginuma H, Kobayashi K (2013) Highly efficient retrograde gene transfer into motor neurons by a lentiviral vector pseudotyped with fusion glycoprotein. PLoS One 8:e75896
Inoue K, Koketsu D, Kato S, Kobayashi K, Nambu A, Takada M (2012) Immunotoxin-mediated tract targeting in the primate brain: selective elimination of the cortico-subthalamic “hyperdirect” pathway. PLoS One 7:e39149
Isa T, Sasaki S (2002) Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol 66:205–241
Isa T, Ohki Y, Seki K, Alstermark B (2006) Properties of propriospinal neurons in the C3–C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J Neurophysiol 95:3674–3685
Ishida A, Isa K, Umeda T, Kobayashi K, Kobayashi K, Hida H, Isa T (2016) Causal link between the cortico-rubral pathway and functional recovery through forced impaired limb use in rats with stroke. J Neurosci 36:455–467
Joshi S, Joshi RL (1996) Molecular biology of human immunodeficiency virus type-1. Transfus Sci 17:351–378
Julien S, Schnichels S, Teng H, Tassew N, Henke-Fahle S, Mueller BK, Monnier PP (2008) Purkinje cell survival in organotypic cultures: implication of Rho and its downstream effector ROCK. J Neurosci Res 86:531–536
Kaibuchi K, Kuroda S, Amano M (1999) Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 68:459–486
Kato S, Inoue K, Kobayashi K, Yasoshima Y, Miyachi S, Inoue S, Hanawa H, Shimada T, Takada M, Kobayashi K (2007) Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein. Hum Gene Ther 18:1141–1151
Kato S, Kobayashi K, Inoue K, Kuramochi M, Okada T, Yaginuma H, Morimoto K, Shimada T, Takada M, Kobayashi K (2011a) A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Hum Gene Ther 22:197–206
Kato S, Kuramochi M, Takasumi K, Kobayashi K, Inoue K, Takahara D, Hitoshi S, Ikenaka K, Shimada T, Takada M, Kobayashi K (2011b) Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein. Hum Gene Ther 22:1511–1523
Kato S, Kuramochi M, Kobayashi K, Fukabori R, Okada K, Uchigashima M, Watanabe M, Tsutsui Y, Kobayashi K (2011c) Selective neural pathway targeting reveals key roles of thalamostriatal projection in the control of visual discrimination. J Neurosci 31:17169–17179
Kato S, Kobayashi K, Kobayashi K (2013) Dissecting circuit mechanisms by genetic manipulation of specific neural pathways. Rev Neurosci 24:1–8
Kato S, Kobayashi K, Kobayashi K (2014) Improved transduction efficiency of a lentiviral vector for neuron-specific retrograde gene transfer by optimizing the junction of fusion envelope glycoprotein. J Neurosci Methods 227:151–158
Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T (2012) Genetic dissection of the circuit for hand dexterity in primates. Nature 487:235–238
Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Fujita K, Kreitman RJ, Pastan I, Nagatsu T (1995) Immunotoxin-mediated conditional disruption of specific neurons in transgenic mice. Proc Natl Acad Sci USA 92:1132–1136
Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K (2004) Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci 24:3480–3488
Kobayashi K, Masuda T, Takahashi M, Miyazaki J, Nakagawa M, Uchigashima M, Watanabe M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K (2011) Rho/Rho-kinase signaling pathway controls axon patterning of a specified subset of cranial motor neurons. Eur J Neurosci 33:612–621
Kobayashi K, Okada K, Kai N (2012) Functional circuitry analysis in rodents using neurotoxins/immunotoxins. In: Alexei M (ed) Neuromethods, controlled genetic manipulations, chapter 10, pp 193–205. Humana Press, New York
Kobayashi K, Kato S, Inoue K, Takada M, Kobayashi K (2016a) Altering entry site preference of lentiviral vectors into neuronal cells by pseudotyping with envelope glycoproteins. Methods Mol Biol 1382:175–186
Kobayashi K, Sano H, Kato S, Kuroda K, Nakamuta S, Isa T, Nambu A, Kaibuchi K, Kobayashi K (2016b) Survival of corticostriatal neurons by Rho/Rho-kinase signaling pathway. Neurosci Lett 630:45–52
LaVail JH, Topp KS, Giblin PA, Garner JA (1997) Factors that contribute to the transneuronal spread of herpes simplex virus. J Neurosci Res 49:485–496
Löw K, Aebischer P, Schneider BL (2013) Direct and retrograde transduction of nigral neurons with AAV6, 8, and 9 and intraneuronal persistence of viral particles. Hum Gene Ther 24:613–629
Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1:173–180
Mair RG, Koch JK, Newman JB, Howard JR, Burk JA (2002) A double dissociation within striatum between serial reaction time and radial maze delayed nonmatching performance in rats. J Neurosci 22:6756–6765
Masamizu Y, Okada T, Kawasaki K, Ishibashi H, Yuasa S, Takeda S, Hasegawa I, Nakahara K (2011) Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience 193:249–258
Massanés-Rotger E, Aldavert-Vera L, Segura-Torres P, Martí-Nicolovius M, Morgado-Bernal I (1998) Involvement of the parafascicular nucleus in the facilitative effect of intracranial self-stimulation on active avoidance in rats. Brain Res 808:220–231
Mentis GZ, Gravell M, Hamilton R, Shneider NA, O’Donovan MJ, Schubert M (2006) Transduction of motor neurons and muscle fibers by intramuscular injection of HIV-1-based vectors pseudotyped with select rabies virus glycoproteins. J Neurosci Methods 157:208–217
Mitrophanous KA, Yoon S, Rohill JB, Patil D, Wilkes FJ, Kim VN, Kingsman SM, Kingsman AJ, Mazarakis ND (1999) Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther 6:1808–1818
Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, Callaway EM, Horowitz MA, Luo L (2011) Cortical representations of olfactory input by trans-synaptic tracing. Nature 472:191–196
Mochizuki H, Schwartz JP, Tanaka K, Brady RO, Reiser J (1998) High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol 72:8873–8883
Naldini L, Blömer U, Gage FH, Trono D, Verma IM (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 93:11382–11388
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43:111–117
Nielsen MH, Pedersen FS, Kjems J (2005) Molecular strategy to inhibit HIV-1 replication. Retrovirology 2:1–20
Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N, Kato S, Kobayashi K, Sakagami M (2015) Double virus vector infection to the prefrontal network of the macaque brain. PLoS One 10:e0132825
Perrelet D, Ferri A, Mackenzie AE, Smith GM, Korneluk RG, Liston P, Sagot Y, Terrado J, Monnier D, Kato AC (2000) IAP family proteins delay motoneuron cell death in vivo. Eur J Neurosci 12:2059–2067
Pluta K, Kacprzak MM (2009) Use of HIV as a gene transfer vector. Acta Biochim Pol 56:531–595
Rabson AB, Martin MA (1985) Molecular organization of the AIDS retrovirus. Cell 40:477–480
Redgrave P, Dean P, Westby GW (1990) Organization of the crossed tecto-reticulo-spinal projection in rat—I. Anatomical evidence for separate output channels to the periabducens area and caudal medulla. Neuroscience 37:571–584
Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M (1996) Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA 93:15266–15271
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4:446–456
Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, Beyer J, Forsayeth J, Bankiewicz KS (2013) Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther 20:348–352
San Sebastian W, Samaranch L, Heller G, Kells AP, Bringas J, Pivirotto P, Forsayeth J, Bankiewicz KS (2013) Adeno-associated virus type 6 is retrogradely transported in the non-human primate brain. Gene Ther 20:1178–1183
Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L (2015) Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524:88–92
Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A (2014) Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81:428–437
Sooksawate T, Isa K, Matsui R, Kato S, Kinoshita M, Kobayashi K, Watanabe D, Kobayashi K, Isa T (2013) Viral vector-mediated selective and reversible blockade of the pathway for visual orienting in mice. Front Neural Circuits 7:162
Stankiewicz TR, Linseman DA (2014) Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci 8:314
Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY (2016) A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92:372–382
Thumkeo D, Watanabe S, Narumiya S (2013) Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol 92:303–315
Tolias KF, Duman JG, Um K (2011) Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol 94:133–148
Ugolini G (2008) Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel) 131:493–506
Van Aelst L, Cline HT (2004) Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol 14:297–304
Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME (2014) Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 344:1250–1255
Wakaizumi K, Kondo T, Hamada Y, Narita M, Kawabe R, Narita H, Watanabe M, Kato S, Senba E, Kobayashi K, Kuzumaki N, Yamanaka A, Morisaki H, Narita M (2016) Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: a study for specific neural control with Gi-DREADD in mice. Mol Pain (in press)
Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM (2007) Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53:639–647
Xu C, Funahashi Y, Watanabe T, Takano T, Nakamuta S, Namba T, Kaibuchi K (2015) Radial glial cell-neuron interaction directs axon formation at the opposite side of the neuron from the contact site. J Neurosci 35:14517–14532
Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S (2003) Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci 23:6759–6767
Zemanick MC, Strick PL, Dix RD (1991) Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc Natl Acad Sci USA 88:8048–8051
Zheng JS, Tang LL, Zheng SS, Zhan RY, Zhou YQ, Goudreau J, Kaufman D, Chen AF (2005) Delayed gene therapy of glial cell line-derived neurotrophic factor is efficacious in a rat model of Parkinson’s disease. Brain Res Mol Brain Res 134:155–161
Zhou X, Vink M, Klaver B, Berkhout B, Das AT (2006) Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther 13:1382–1390
Acknowledgements
This work was supported by a grant-in-aid for Scientific Research on Innovative Areas “Adaptive Circuit Shift” (26112002) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Dr. A. Nambu, T. Isa, M. Takada, and K. Kaibuchi for valuable discussions; Dr. Ira Pastan for providing the recombinant immunotoxin; and St. Jude Children’s Research Hospital (Dr. A. Nienhuis) and George Washington University for providing the HIV-1-based vector system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, K., Kato, S. & Kobayashi, K. Genetic manipulation of specific neural circuits by use of a viral vector system. J Neural Transm 125, 67–75 (2018). https://doi.org/10.1007/s00702-016-1674-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1674-7