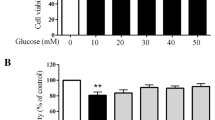
Abstract
Alzheimer’s disease (AD) is the most common form of dementia in the elderly. Accumulation of Aβ peptides in the brain has been suggested as the cause of AD (amyloid cascade hypothesis); however, the mechanism for the abnormal accumulation of Aβ in the brains of AD patients remains unclear. A plethora of evidence has emerged to support a link between metabolic disorders and AD. This study was designed to examine the relationship between energy status and Aβ production. Neuro 2a neuroblastoma cells overexpressing human amyloid precursor protein 695 (APP cells) were cultured in media containing different concentrations of glucose and agonist or antagonist of AMP-activated-protein-kinase (AMPK), a metabolic master sensor. The results showed that concentrations of glucose in the culture media were negatively associated with the activation statuses of AMPK in APP cells, but positively correlated with the levels of secreted Aβ. Modulating AMPK activities affected the production of Aβ. If APP cells were cultured in high glucose medium (i.e., AMPK was inactive), stimulation of AMPK activity decreased the production levels of Aβ. On the contrary, if APP cells were incubated in medium containing no glucose (i.e., AMPK was activated), inhibition of AMPK activity largely increased Aβ production. As AMPK activation is a common defect in metabolic abnormalities, our study supports the premise that metabolic disorders may aggravate AD pathogenesis.





Similar content being viewed by others
References
Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M (2010) Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 75:1195–1202
Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61:661–666
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14:750–756
Burkewitz K, Zhang Y, Mair WB (2014) AMPK at the nexus of energetics and aging. Cell Metab 20:10–25
Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF (2009) Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA 106:3907–3912
Chiang MC, Cheng YC, Chen HM, Liang YJ, Yen CH (2014) Rosiglitazone promotes neurite outgrowth and mitochondrial function in N2A cells via PPARgamma pathway. Mitochondrion 14:7–17
Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA (2011) Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res Rev 10:264–273
Craft S (2009) The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 66:300–305
Culmsee C, Monnig J, Kemp BE, Mattson MP (2001) AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17:45–58
de la Monte SM, Tong M, Lester-Coll N, Plater JM, Wands JR (2006) Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis 10:89–109
Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX (2009) Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer’s disease. Am J Pathol 175:2089–2098
Dermaku-Sopjani M, Abazi S, Faggio C, Kolgeci J, Sopjani M (2014) AMPK-sensitive cellular transport. J Biochem 155:147–158
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Hardy J (2006) Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9:151–153
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol 490:115–125
Hurtado-Carneiro V, Roncero I, Blazquez E, Alvarez E, Sanz C (2013) PAS kinase as a nutrient sensor in neuroblastoma and hypothalamic cells required for the normal expression and activity of other cellular nutrient and energy sensors. Mol Neurobiol 48:904–920
Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53:474–481
Jin J, Mullen TD, Hou Q, Bielawski J, Bielawska A, Zhang X, Obeid LM, Hannun YA, Hsu Y-T (2009) AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res 50:2389–2397
Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E (2010) Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp Neurol 223:422–431
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84
Kawashima J, Alquier T, Tsuji Y, Peroni OD, Kahn BB (2012) Ca2 +/calmodulin-dependent protein kinase kinase is not involved in hypothalamic AMP-activated protein kinase activation by neuroglucopenia. PLoS ONE 7:e36335
Ke YD, Delerue F, Gladbach A, Gotz J, Ittner LM (2009) Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 4:e7917
Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA (2003) AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31:162–168
Kim KW, Jhoo JH, Lee JH, Lee DY, Lee KU, Youn JY, Woo JI (2001) Transferrin C2 variant does not confer a risk for Alzheimer’s disease in Koreans. Neurosci Lett 308:45–48
Kim HS, Moon S, Paik JH, Shin DW, Kim LS, Park CS, Ha J, Kang JH (2015) Activation of the 5′-AMP-activated protein kinase in the cerebral cortex of young senescence-accelerated P8 mice and association with GSK3β- and PP2A-dependent inhibition of p-tau396 expression. J Alzheimers Dis. doi:10.3233/JAD-150035
Kojima N, Kurosawa N, Nishi T, Hanai N, Tsuji S (1994) Induction of cholinergic differentiation with neurite sprouting by de novo biosynthesis and expression of GD3 and b-series gangliosides in Neuro 2a cells. J Biol Chem 269:30451–30456
Lee CW, Shih YH, Wu SY, Yang T, Lin C, Kuo YM (2013) Hypoglycemia induces tau hyperphosphorylation. Curr Alzheimer Res 10:298–308
Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, Yan JC, Zhou HD (2011) Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76:1485–1491
Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX (2008) Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 582:359–364
Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R (2001) Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154:635–641
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 280:37377–37382
Mazza M, Marano G, Traversi G, Bria P, Mazza S (2011) Primary cerebral blood flow deficiency and Alzheimer’s disease: shadows and lights. J Alzheimers Dis 23:375–389
Merrill GF, Kurth EJ, Hardie DG, Winder WW (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273:E1107–E1112
Messier C (2005) Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 26:26–30
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574
Mosconi L, Pupi A, De Leon MJ (2008) Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Annal New York Acad Sci 1147:180–195
Paris D, Town T, Mori T, Parker TA, Humphrey J, Mullan M (2000) Soluble beta-amyloid peptides mediate vasoactivity via activation of a pro-inflammatory pathway. Neurobiol Aging 21:183–197
Ropelle ER, Pauli JR, Zecchin KG, Ueno M, de Souza CT, Morari J, Faria MC, Velloso LA, Saad MJ, Carvalheira JB (2007) A central role for neuronal adenosine 5′-monophosphate-activated protein kinase in cancer-induced anorexia. Endocrinology 148:5220–5229
Russell RR 3rd, Bergeron R, Shulman GI, Young LH (1999) Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277:H643–H649
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118:460–474
Schubert D (2005) Glucose metabolism and Alzheimer’s disease. Ageing Res Rev 4:240–257
Sims-Robinson C, Kim B, Rosko A, Feldman EL (2010) How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 6:551–559
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T (2013) Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem 288:37216–37229
Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L (2014) Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging 35:941–957
Thomas T, Thomas G, McLendon C, Sutton T, Mullan M (1996) beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380:168–171
Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D (2011) AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid β-peptide exposure. Biochem J 434:503–512
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 285:9100–9113
Vingtdeux V, Davies P, Dickson DW, Marambaud P (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol 121:337–349
Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K (2005) Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330:1360
Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71:1057–1064
Won JS, Im YB, Kim J, Singh AK, Singh I (2010) Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochem Biophys Res Commun 399:487–491
Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472:230–233
Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2011) Midlife overweight and obesity increase late-life dementia risk. Neurology 76:1568–1574
Yang TT, Hsu CT, Kuo YM (2009) Amyloid precursor protein, heat-shock proteins, and Bcl-2 form a complex in mitochondria and modulate mitochondria function and apoptosis in N2a cells. Mech Ageing Dev 130:592–601
Yoshida H, Goedert M (2012) Phosphorylation of microtubule-associated protein tau by AMPK-related kinases. J Neurochem 120:165–176
Acknowledgments
This study was supported by grants from Ministry of Science and Technology of Taiwan to YMK (101-2320-B-006-006-MY3) and TTY (102-2314-B-214-006-MY3). We thank Ms. Tiffany Hu for reading and commenting on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, TT., Shih, YS., Chen, YW. et al. Glucose regulates amyloid β production via AMPK. J Neural Transm 122, 1381–1390 (2015). https://doi.org/10.1007/s00702-015-1413-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-015-1413-5