Abstract
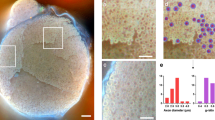
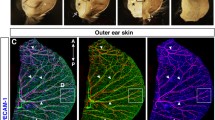
Peripheral neuropathy is a devastating complication of diabetes conferring vast morbidity and mortality. Despite prolonged efforts to elucidate the mechanisms underlying diabetic related neuropathic phenomena and develop effective therapies, current treatment is for the most part glycemic control and symptomatic care. This is partially due to the intricate pathophysiology of diabetic neuropathy and the scarcity of valid experimental models. The aim of the study was to establish novel systems enabling monitoring and dissection of significant processes in the development of diabetic neuropathy. In a non-invasive in vivo model, two-photon microscopy is applied to evaluate mechanoreceptors (Meissner corpuscles) within an intact footpad of transgenic mice expressing a fluorescent neuronal tracer. By applying this advanced technology, which couples potent tissue penetration with superb resolution, we documented qualitative and quantitative diabetes-specific alterations in these sensory structures. Detection of such changes previously required laborious invasive histopathological techniques. In parallel, we present an ex vivo system that mimics the native microenvironment of the nerve ending via a unique co-culture of primary sensory neurons and thin skin slices. In conjunction with innovative high-throughput digital axonal measurements and computerized quantification tools, this method enables an unbiased exploration of neuronal autonomous and non-autonomous malfunctions. Using this setup we demonstrate that while the diabetic nerve retains a near-normal growth and regeneration capacities, the diabetic skin exhibits a decreased ability to support axonal outgrowth. Thus, an early target organ failure rather than intrinsic neuronal failure may initiate the neuropathy. Overall, the illustrated experimental platforms may greatly facilitate the holistic investigation of diabetic neuropathy.




Similar content being viewed by others
References
Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV (1996) The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med 2:703–707
Apfel SC (2002) Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 50:393–413
Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, Glazner GW, Fernyhough P, Zochodne DW (2008) Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes 57:1664–1673
Calcutt NA, Cooper ME, Kern TS, Schmidt AM (2009) Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 8:417–429
Chen YS, Chung SS, Chung SK (2005) Noninvasive monitoring of diabetes-induced cutaneous nerve fiber loss and hypoalgesia in thy1-YFP transgenic mice. Diabetes 54:3112–3118
Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B (1987) Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Nat Acad Sci USA 84:6596–6600
Diamond J, Foerster A, Holmes M, Coughlin M (1992) Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J Neurosci Off J Soc Neurosc 12:1467–1476
Dyck PJ (2007) Enumerating Meissner corpuscles: future gold standard of large fiber sensorimotor polyneuropathy? Neurology 69:2116–2118
Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28:41–51
Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ (2008) Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci 49:2635–2642
Guz Y, Nasir I, Teitelman G (2001) Regeneration of pancreatic beta cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology 142:4956–4968
Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL et al (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104
Hellweg R, Raivich G, Hartung HD, Hock C, Kreutzberg GW (1994) Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol 130:24–30
Herrmann DN, Boger JN, Jansen C, Alessi-Fox C (2007) In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology 69:2121–2127
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Lindsay RM (1988) Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci Off J Soc Neurosci 8:2394–2405
Nolano M, Provitera V, Lullo F, Saltalamacchia A, Crisci C, Lanzillo B, Santoro L (2001) Tactile stimulation and mechanoreceptors in sensory neuropathies. Neurol Sci 22:S31–S36
Obrosova IG (2009) Diabetes and the peripheral nerve. Biochim Biophys Acta 1792:931–940
Pare M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL (2001) The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. J Neurosci 21:7236–7246
Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC (2004) The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain J Neurol 127:1606–1615
Ras VR, Nava PB (1986) Age-related changes of neurites in Meissner corpuscles of diabetic mice. Exp Neurol 91:488–501
Said G (2007) Diabetic neuropathy—a review. Nat Clin Pract Neurol 3:331–340
Sullivan KA, Feldman EL (2005) New developments in diabetic neuropathy. Curr Opin Neurol 18:586–590
Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH (2005) Vascular risk factors and diabetic neuropathy. N Engl J Med 352:341–350
Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9:36–45
Vinik AI, Mehrabyan A (2004) Diabetic neuropathies. Med Clin North Am 88:947–999 xi
Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M (2005) Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45:513–523
Zochodne DW (2007) Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve 36:144–166
Zochodne DW (2008) Diabetic polyneuropathy: an update. Curr Opin Neurol 21:527–533
Acknowledgments
We are grateful to Dr. Guy Shakhar (Depratment of Immunology, the Weizmann Institute) and Mr. Yosef Addadi (Department of Biological Regulation, the Weizmann Institute) for their help with the two-photon microscopy and anesthesia, Dr. Rony Paz (Department of Neurobiology, the Weizmann Institute) for the statistical analysis and Dr. J Sanse, (Department of Cellular and Molecular Biology, Harvard University) for the YFP-16 mouse line. The research was funded by the Nella and Leon Benoziyo Center for Neurological Diseases of the Weizmann Institute.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
702_2012_808_MOESM1_ESM.pdf
Supplementary figure 1 Whole embryonic dorsal root ganglia (DGR) explants were isolated and grown either alone (a) or in the presence of NGF (b) or healthy skin (d). NGF neutralizing antibodies abolished the NGF-induced axonal growth, yet not the skin-induced axonal growth (c and e respectively) (PDF 6123 kb)
702_2012_808_MOESM2_ESM.tiff
Supplementary figure 2 Digital assessment of Meissner corpuscles: a. Serial footpad acquisitions are stacked to create a 2D image of the 3D projection. b. An automatic digital threshold is activated to enhance bright areas. c. Particle analysis tool is applied to sum the total square pixels of above-threshold intense areas (TIFF 1521 kb)
Supplementary video clips Pad clips a and b depict a 360º view of a 3D reconstruction of two-photon images of footpads (3rd pad of right hind limb) of control (clip a) or diabetic (clip b) mice sacrificed immediately before acquisition. Pad clip c depicts a reconstruction of a two-photon acquisition performed on a live anesthetized mouse (MPG 602 kb)
Supplementary material 4 (MPG 460 kb)
Supplementary material 5 (MPG 1170 kb)
Rights and permissions
About this article
Cite this article
Amit, S., Yaron, A. Novel systems for in vivo monitoring and microenvironmental investigations of diabetic neuropathy in a murine model. J Neural Transm 119, 1317–1325 (2012). https://doi.org/10.1007/s00702-012-0808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0808-9