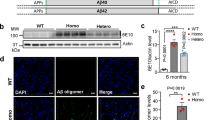
Abstract
Alzheimer’s disease (AD) is the most common origin of dementia in the elderly. Although the cause of AD remains unknown, several factors have been identified that appear to play a critical role in the development of this debilitating disorder. In particular, amyloid precursor protein (APP), tau hyperphosphorylation, and the secretase enzymes, have become the focal point of recent research. Over the last two decades, several transgenic and non-transgenic animal models have been developed to elucidate the mechanistic aspects of AD and to validate potential therapeutic targets. Transgenic rodent models over-expressing human β-amyloid precursor protein (β-APP) and mutant forms of tau have become precious tools to study and understand the pathogenesis of AD at the molecular, cellular and behavioural levels, and to test new therapeutic agents. Nevertheless, none of the transgenic models of AD recapitulate fully all of the pathological features of the disease. Octodon degu, a South American rodent has been recently found to spontaneously develop neuropathological signs of AD in old age. This review aims to address the limitations and clinical relevance of transgenic rodent models in AD, and to highlight the potential for O. degu as a natural model for the study of AD neuropathology.



Similar content being viewed by others
References
Abraham C, Selkoe D, Potter H, Price D, Cork L (1989) α1-Antichymotrypsin is present together with the β-protein in monkey brain amyloid deposits. Neuroscience 32:196–198
Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K (2004) Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279:34873–34881
Alonso Adel C, Li B, Grundke-Iqbal I, Iqbal K (2006) Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA 103:8864–8869
Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2:783–787
Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K (2001) Interaction of tau isoforms with Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem 276:37967–37973
Ardiles A, Barrientos S, Araya J, Tapia Rojas C, Inestrosa N, Kirkwood A, Palacios A (2011) β-Amyloid dodecamers and hyperphosphorylated Tau correlates with synaptic and cognitive impairments in aged Octodon degus. Paper presented at the Reunión Anual Sociedad Chilena de Neurociencia, Las Cruces, Chile
Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ (2000) Microglia, amyloid and dementia in alzheimer disease: a correlative study. Neurobiol Aging 21:39–47
Arendt T, Stieler J, Strijkstra A, Hut R, Rudiger J (2003) Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 23:6972–6981
Asberom T, Zhao Z, Bara TA, Clader JW, Greenlee WJ, Hyde LA, Josien HB, Li W, McPhail AT, Nomeir AA, Parker EM, Rajagopalan M, Song L, Wong GT, Zhang L, Zhang Q, Pissarnitski DA (2007) Discovery of gamma-secretase inhibitors efficacious in a transgenic animal model of Alzheimer’s disease. Bioorg Med Chem Lett 17:511–516
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27:9115–9129
Atamna H, Frey WH II, Ko N (2009) Human and rodent amyloid-β peptides differentially bind heme: Relevance to the human susceptibility to Alzheimer’s disease. Arch Biochem Biophys 487:59–65
Bate C, Boshuizen RS, Langeveld JP, Williams A (2002) Temporal and spatial relationship between the death of PrP-damaged neurones and microglial activation. Neuroreport 13:1695–1700
Benatar M, Polak M, Kaplan S, Glass J (2006) Preventing familial amyotrophic lateral sclerosis: is a clinical trial feasible? J Neurol Sci 251:3–9
Bons N, Mestre N, Petter A (1991) Senile plaques and neurofibrillary changes in the brain of aged lemurian primate, Microcebus murinus. Neurobiol Aging 13:99–105
Bons N, Mestre N, Richie K, Petter A, Podlisny M, Selkoe D (1994) Identification of amyloid beta protein in the brain of the small, short-lived lemurian primate Microcebus marinus. Neurobiol Aging 15:215–220
Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH (2006) Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer’s disease? Genes Brain Behav 5:120–130
Boutajangout A, Quartermain D, Sigurdsson EM (2010) Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci 30:16559–16566
Brendza RP, Bales KR, Paul SM, Holtzman DM (2002) Role of apoE/Aβ interactions in Alzheimer’s disease: insights from transgenic mouse models. Mol Psychiatry 7:132–135
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A (1998) Neuron loss in APP transgenic mice. Nature 395:755–756
Carrodeguas J, Rodolosse A, Garza M, Sanz-Clemente A, Perez-Pe R, Lacosta A, Dominguez L, Monleon I, Sanchez-Diaz R, Sorribas V, Sarasa M (2005) The chick embryo appears as a natural model for research in beta-amyloid precursor protein processing. Neuroscience 134:1285–1300
Chan AW (2004) Transgenic nonhuman primates for neurodegenerative diseases. Reprod Biol Endocrinol 2:39
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676
Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276:21562–21570
Chu W, Qian C (2005) Expressions of Aβ1–40, Aβ1–42, tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats. Di Yi Jun Yi Da Xue Xue Bao 25:168–170
Dani S (1997) Mechanisms of aging: a survey. In: Dani S, Hori A, Walter G (eds) Principles of neural aging. Elsevier, Amsterdam, pp 5–18
De La Monte SM, Wands J (2005) Review of insulin and insulin-like growth factor expression, signalling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis 7:45–61
Dedeoglu A, Cormier K, Payton S, Tseitlin KA, Kremsky JN, Lai L, Li X, Moir RD, Tanzi RE, Bush AI, Kowall NW, Rogers JT, Huang X (2004) Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer’s amyloidogenesis. Exp Gerontol 39:1641–1649
Delacourte A (1990) General and dramatic glial reaction in Alzheimer brains. Neurology 40:33–37
Delacourte A, Buee L (2005) Animal models of Alzheimer’s disease: a road full of pitfalls. Psychol Neuropsychiatr Vieil 3:261–270
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90
Dodart JC, Mathis C, Bales KR, Paul SM (2002) Does my mouse have Alzheimer’s disease? Genes Brain Behav 1:142–155
Du P, Wood K, Rosner M, Cunningham D, Tate B, Georghegan K (2007) Dominance of amyloid precursor protein sequence over host cell secretases in determining beta-amyloid profiles studies of interspecies variation and drug action by internally standardised immunoprecipitation/mass spectrometry. J Pharmacol Exp Ther 320:1144–1152
Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of gamma-secretase activity. Nat Cell Biol 5:486–488
Feng Z, Cheng Y, Zhang JT (2004) Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res 37:198–206
Ferri CP, Prince M, Brayne C, Brodaty C, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y (2006) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117
Flood D, Howland D, Lin Y-G, Ciallella J, Trusko S, Scott R, Savage M (2003) Aβ deposition in a transgenic rat model of Alzheimer’s disease. Poster 84222. Society for Neuroscience meeting
Flood D, Lin YG, Lang DM, Trusko SP, Hirsch JD, Savage MJ, Scott RW, Howland DS (2007) A transgenic rat model of Alzheimer’s disease with extracellular Amyloid-beta deposition. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2007.10.006
Galimberti D, Scarpini E (2011) Disease-modifying treatments for Alzheimer’s disease. Ther Adv Neurol Disord 4:203–216
Gearing M, Rebeck G, Hyman B, Tigges J, Mirra S (1994) Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer’s disease. PNAS 91:9382–9386
Gearing M, Tigges J, Mori H, Mirra S (1996a) Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol Aging 17:903–906
Gearing M, Mori H, Mirra S (1996b) Aβ peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol 39:395–399
Gearing M, Tigges J, Mori H, Mirra S (1997) β-Amyloid (Aβ) deposition in the brains of aged orangutans. Neurobiol Aging 18:139–146
German DC, Eisch AJ (2004) Mouse models of Alzheimer’s disease: insight into treatment. Rev Neurosci 15:353–369
Ghribi O, Golovko M, Larsen B, Schrag M, Murphy E (2006) Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol enriched diets. J Neurochem 99:438–449
Glabe C (2001) Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer’s disease. J Mol Neurosci 17:137–145
Glabe C (2006) Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging 27:570–575
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterisation of a novel cerebrovascular amyloid protein. Biochem Biophys Res Comm 120:885–890
Golde TE, Younkin SG (2001) Presenilins as therapeutic targets for the treatment of Alzheimer’s disease. Trends Mol Med 7:264–269
Goldman-Rakic P, Brown R (1981) Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience 6:177–187
Gonzalo-Ruiz A, Gonzalez I, Sanz-Anquela J (2003) Effects of beta-amyloid protein on serotoninergic, noradrenergic, and cholinergic markers in neurons of the pontomesencephalic tegmentum in the rat. J Chem Neuroanat 26:153–169
Gotz J, Ittner LM (2008) Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci 9:532–544
Gotz J, Nitsch RM (2001) Compartmentalized tau hyperphosphorylation and increased levels of kinases in transgenic mice. Neuroreport 12:2007–2016
Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ 42 fibrils. Science 293:1491–1495
Gotz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinski P, Chen F (2004) Transgenic animal models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry 9:664–683
Gotz J, Ittner LM, Kins S (2006) Do axonal defects in tau and amyloid precursor protein transgenic animals model axonopathy in Alzheimer’s disease? J Neurochem 98:993–1006
Grieb P, Kryczka T, Fiedorowicz M, Frontczak-Baniewicz M, Walski M (2004) Expansion of the Golgi apparatus in rat cerebral cortex following intracerebroventricular injections of streptozotocin. Acta Neurobiol Exp (Wars) 64:481–489
Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S (2006) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem
Gunawardena S, Goldstein LS (2001) Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32:389–401
Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, MP M (2007) Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 26:212–220
Hall GF, Lee VM, Lee G, Yao J (2001) Staging of neurofibrillary degeneration caused by human tau overexpression in a unique cellular model of human tauopathy. Am J Pathol 158:235–246
Hamann S, Monarch ES, Goldstein FC (2002) Impaired fear conditioning in Alzheimer’s disease. Neuropsychologia 40:1187–1195
Hardy J (2006) Has the amyloid cascade hypothesis of Alzheimer’s disease been proved. Curr Alzheimer Res 3:71–73
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185
Hardy J, Selkoe D (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hare B, Brown M, Williamson C, Tomasello M (2002) The domestication of social cognition in dogs. Science 298:1634–1636
Harper J, Wong S, Lieber C, Lansbury P (1997) Observation of metastable Aβ-amyloid protofibrils by atomic force microscopy. Chem Biol 4:119–125
Hartig W, Bruckner G, Schmidt C, Brauer K, Bodewitz G, Turner J, Bigl V (1997) Co-localisation of β-amyloid peptides, apolipoprotein E and glial markers in senile plaques in the prefrontal cortex of old rhesus monkeys. Brain Res 751:315–322
Hartig W, Klein C, Brauer K, Schuppel K, Arendt T, Bruckner G, Bigl V (2000) Abnormally phosphorylated protein tau in the cortex of aged individuals of various mammalian orders. Acta Neuropathol 100:305–312
Hartig W, Goldhammer S, Bauer U, Wegner F, Wirths O, Bayer T, Grosche J (2010) Concomitant detection of β-amyloid peptides with N-terminal truncation and different C-terminal endings in cortical plaques from cases with Alzheimer’s disease, senile monkeys and triple transgenic mice. J Chem Neuroanat 40:82–92
Hashimoto M, Rockenstein E, Crews L, Masliah E (2003) Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol Med 4:21–36
Higgins GA, Jacobsen H (2003) Transgenic mouse models of Alzheimer’s disease: phenotype and application. Behav Pharmacol 14:419–438
Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Sartorious LJ (1999) Expression of human apolipoprotein E reduces amyloid beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest 103:R15–R21
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, LJ S (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 97:2892–2897
Howlett DR, Richardson JC (2009) The pathology of APP transgenic mice: a model of Alzheimer’s disease or simply overexpression of APP? Histol Histopathol 24:83–100
Hoyer S (2000) Brain glucose and energy metabolism abnormalities in sporadic Alzheimer’s disease. Causes and consequences: an update. Exp Gerontol 35:1363–1372
Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer’s disease. Euro J Pharmacol 490:115–125
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Hussain I, Hawkins J, Harrison D, Hille C, Wayne G, Cutler L, Buck T, Walter D, Demont E, Howes C, Naylor A, Jeffrey P, Gonzalez MI, Dingwall C, Michel A, Redshaw S, Davis JB (2007) Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases beta-cleavage of amyloid precursor protein and amyloid-beta production in vivo. J Neurochem 100:802–809
Hydea LA, Kazdobaa TM, Grillia M, Lozzaa G, Brussaa R, Zhanga Q, Wonga GT, McCoola MF, Zhanga L, Parkera EM, Higginsa GA (2005) Age-progressing cognitive impairments and neuropathology in transgenic CRND8 mice. Behav Brain Res 160:344–355
Inestrosa NC, Reyes AE, Chacon MA, Cerpa W, Villalon A, Montiel J, Merabachvili G, Aldunate R, Bozinovic F, Aboitiz F (2005) Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol Aging 26:1023–1028
Jawhar S, Wirths O, Schilling S, Graubner S, Demuth H-U, Bayer T (2011) Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioural deficits, and glutaminyl cyclase knock-out rescues the behavioural phenotype in 5×FAD mice. J Bio Chem 286:4454–4460
Johnson S, Lampert-Etchells M, Pasinetti G, Rozovsky I, Finch C (1992) Complement mRNA in the mammalian brain: responses to Alzheimer’s disease and experimental brain lesioning. Neurobiol Aging 13:641–648
Johnstone E, Chaney M, Norris F, Pascual R, Little S (1991) Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res 10:299–305
Jolas T, Zhang XS, Zhang Q, Wong G, Del Vecchio R, Gold L, Priestley T (2002) Long-term potentiation is increased in the CA1 area of the hippocampus of APPswe/indCRND8mice. Neurobiol Dis 11:394–409
Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) APP processing and synaptic function. Neuron 37:925–937
Kanemaru K, Iwatsubo T, Ihara Y (1996) Comparable amyloid β-protein (Aβ) 42(43) and Aβ40 deposition in aged monkey brain. Neurosci Lett 214:196–198
Kelly PH, Bondolfi L, Hunziker D, Schlecht H, Carver K, Maguire E, Abramowski D, Wiederhold K, Sturchler-Pierrat C, Jucker M, Bergmanna R, Staufenbiel M, Sommera B (2003) Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol Aging 24:365–378
Kiatipattanasakul W, Nakayama H, Yongsiri S, Chotiapisitkul S, Nakamura S, Kojima H, Doi K (2000) Abnormal neuronal and glial argyrophilic fibrillary structures in the brain of aged individuals of various mammalian orders. Acta Neuropathol 100:305–312
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179
Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski J, Schellenberg GD (2003) From the cover: neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. PNAS 100:9980–9985
Kulic L, Kurosinski P, Chen F, Tracy J, Mohajeri MH, Li H, Nitsch RM, Gotz J (2006) Active immunization trial in Aβ42-injected P301L tau transgenic mice. Neurobiol Dis 22:50–56
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M (2000) Neurofibrillary tangles, amyotrophy and passive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25:402–405
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant Tau and APP. Science 293:1487–1491
Li T (2004) Recent progress in Alzheimer’s disease: animal models lead the way. Drug Discov Today Dis Models 1:145–149
Li L, Cao D, Kim H, Lester R, Fukuchi K (2006) Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol 60:729–739
Liberski P, Sikorska B, Bratosiewicz-Wasik J, Gajdusek D, Brown P (2004) Neuronal cell death in transmissible spongiform encephalopathies (prion diseases) revisited: from apoptosis to autophagy. Int J Biochem Cell Biol 36:2473–2490
Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr, Frautschy SA, GM C (2005) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci 25:3032–3040
Lopez EM, Bell KFS, Ribeiro-da-Silva A, Cuello AC (2004) Early changes in the neurons of the hippocampus and neocortex in transgenic rats expressing intracellular human Aβ. J Alzheimers Dis 6:421–431
Maccioni RB, Munoz JP, Barbeito L (2001) The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch Med Res 32:367–381
Martin L, Sisodia S, Koo E, Cork L, Dellovade T, Weidemann A, Beyreuther K, Masters C, Price D (1991) Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci USA 88:1461–1465
Masters CL, Simms G, Weinman NA, Maulthaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer’s disease and Downs syndrome. Proc Natl Acad Sci USA 82:4245–4249
Mattson MP (2004) Pathway towards and away from Azheimer’s disease. Nature 430:630–639
Mesulam MM, Geula C (1988) Acetylcholinesterase-rich pyramidal neurons in the human neocortex and hippocampus: absence at birth, development during the life span, and dissolution in Alzheimer’s disease. Ann Neurol 24:765–773
Mina E, Demuro A, Kayed R, Milton S, Parker I, Glabe C (2004) Membrane disruption and elevated intracellular calcium as a common mechanism of amyloid oligomer-induced neurodegeneration. Neurosci Abstr. 449:20
Minamide L, Striegel A, Boyle J, Meberg P, Bamburg J (2000) Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol 2:628–636
Morgan B (1990) Complement. Clinical aspects and relevance to disease. Academic Press, San Diego
Nakumura S, Tamaoka A, Sawamura N, Shoji S, Nakayama H, Ono F, Sakakibara I, Yoshikawa Y, Mori H, Goto N, Doi K (1995) Carboxyl end-specific monoclonal antibodies to amyloid β protein (Aβ) subtypes (Aβ40 and Aβ42(43)) differentiate Aβ in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci Lett 201:151–154
Nicholl JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO (2003) Neuropathology of human Alzheimer’s disease after immunisation with amyloid-beta peptide: a case report. Nat Med 9:448–452
Nichols D, Day J, Laping N, Johnson S, Finch C (1993) GFAP mRNA increases with age in rat and human tissue. Neurobiol Aging 14:421–429
Nikolic WV, Bai Y, Obregon D, Hou H, Mori T, Zeng J, Ehrhart J, Shytle RD, Giunta B, Morgan D, Town T, Tan J (2007) Transcutaneous beta-amyloid immunization reduces cerebral beta-amyloid deposits without T cell infiltration and microhemorrhage. Proc Natl Acad Sci USA 104:2507–2512
Oakley H, Cole S, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Eldik L, Berry R, Vassar R (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuronal loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26:10129–10140
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39:409–421
Pathan A, Viswanad B, Sonkusare S, Ramarao P (2006) Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci 79:2209–2216
Pennanen L, Welzl H, D’Adamo P, Nitsch RM, Gotz J (2004) Accelerated extinction of conditioned taste aversion in P301L tau transgenic mice. Neurobiol Dis 15:500–509
Permanne B, Adessi C, Saborio GP, Fraga S, Frossard MJ, Van Dorpe J (2002) Reduction of amyloid load and cerebral damage in a transgenic mouse model of Alzheimer’s disease by treatment with a beta-sheet breaker peptide. FASEB J 16:860–862
Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M (2002) Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science 298:1379
Podlisny M, Tolan D, Selkoe D (1991) Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer’s disease. Am J Pathol 138:1423–1435
Poduri A, Gearing M, Rebeck G, Mirra S, Tigges J, Hyman B (1994) Apolipoprotein E4 and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. Am J Pathol 144:1183–1187
Poirier R, Wolfer DP, Welzl H, Tracy J, Galsworthy MJ, Nitsch RM, Mohajeri MH (2006) Neuronal neprilysin overexpression is associated with attenuation of Aβ-related spatial memory deficit. Neurobiol Dis 24:475–483
Ponce A, Cerpa W, Muñoz P, Inestrosa N, Palacios A (2007) Aging and spatial memory in the rodent Octodon degus. In: IIIrd Neurotoxicity Society meeting, Pucon, Chile, April 2007
Popovic N, Bano-Otalora B, Rol MA, Caballero-Bleda M, Madid JA, Popovic M (2009) Aging and time-of-day effects on anxiety in female Octodon degus. Behav Brain Res 200:117–121
Prasad CV, Zheng M, Vig S, Bergstrom C, Smith DW, Gao Q, Yeola S, Polson CT, Corsa JA, Guss VL, Loo A, Wang J, Sleczka BG, Dangler C, Robertson BJ, Hendrick JP, Roberts SB, Barten DM (2007) Discovery of (S)-2-((S)-2-(3,5-difluorophenyl)-2-hydroxyacetamido)-N-((S,Z)-3-methyl-4-oxo-4,5-dihydro-3H-benzo[d][1,2]diazepin-5-yl)propanamide (BMS-433796): a gamma-secretase inhibitor with Aβ lowering activity in a transgenic mouse model of Alzheimer’s disease. Bioorg Med Chem Lett
Prickaerts J, Fahrig T, Blokland A (1999) Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an i.c.v. injection of streptozocin: a correlation analysis. Behav Brain Res 102:73–88
Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 3:905–912
Puglielli L, Tanzi RE, Kovacs DM (2003) Alzheimer’s disease: the cholesterol connection. Nat Neurosci 6:345–351
Pugliese MJM, Mahy N, Ferrer I (2006) Diffuse beta-amyloid plaques and hyperphosphorylated tau are unrelated processes in aged dogs with behavioural deficits. Acta Neuropathol (Berl) 112:175–183
Rahman A, Ting K, Cullen KM, Brew BJ, Braidy N, Guillemin GJ (2009) The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE 4:e6344
Rapp P, Amaral D (1992) Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci 15:340–345
Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J (2005) Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25:8807–8814
Richards JG, Higgins GA, Ouagazzal AM, Ozmen L, Kew JNC, Bohrmann B, Malherbe P, Brockhaus M, Loetscher H, Czech C, Huber G (2003) PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J Neurosci 23:8989–9003
Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL (2003) Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60:1685–1691
Rocchi A, Pellegrini S, Siciliano G, Mum L (2003) Causative and susceptibility genes for Alzheimer’s disease: a review. Brain Res Bull 61:1–24
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E (2006) Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. J Neurosci Res 83:1252–1261
Rofina J, van Andel I, van Ederen A, Papaioannou N, Yamaguchi H, Guys E (2000) Canine counterpart of senile dementia of the Alzheimer type: amyloid plaques near capillaries but lack of spatial relationship with activated microglia and macrophages. Amyloid: J Protein Folding Disord 10:86–96
Russell M, White R, Patel E, Markesbery W, Watson C, Geddes J (1992) Familial influence on plaque formation in the beagle brain. Neuroreport 3:1093–1096
Saido T, Iwatsubo T, Mann D, Shimada H, Ihara Y, Kawashima S (1995) Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron 14:457–466
Saido T, Yamao-Harigaya W, Iwatsubo T, Kawashima S (1996) Amino- and carboxyl-terminal heterogeneity of β-amyloid peptides deposited in human brain. Neurosci Lett 215:173–176
Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P (2006) Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to insulin signalling pathway. J Neurochem 96:1005–1015
Salvin H, McGreevy P, Sachdev P, Valenzuela M (2011a) The canine sand maze: an appetitive spatial memory paradigm sensitive to age-related change in dogs. J Exp Anal Behav 95:109–118
Salvin H, McGreevy P, Sachdev P, Valenzuela M (2011b) The canine cognitive dysfunction rating scale (CCDR): a data-driven and ecologically relevant assessment tool. Vet J 188:331–336
Sarasa M, Gallego M (2006) Alzheimer-like neurodegeneration as a probable cause of cetacean stranding. In: 5th forum of European neuroscience, Vienna
Sarasa M, Pesini P (2009) Natural non-transgenic animal models for research in Alzheimer’s disease. Curr Alzheimer Res 6:171–178
Satou T, Cummings B, Head E, Nielson K, Hahn F, Milgram N (1997) The progression of beta-amyloid deposition in the frontal cortex of aged canines. Brain Res 774:35–43
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177
Schenk DB, Seubert P, Grundman M, Black R (2005) A beta immunotherapy: lessons learned for potential treatment of Alzheimer’s disease. Neurodegener Dis 2:255–260
Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y (1995) Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci USA 92:1989–1993
Schultz C, Braak E, Braak H (1998) A nonhuman primate model for tau-positive cytoskeletal pathology affecting neurons astrocytes and oligodendrocytes. Clin Neuropathol 17:251
Schultz C, Dehghani F, Hubbard G, Thal D, Struckhoff G, Braak E, Braak H (2000a) Filamentous tau pathology in nerve cells, astrocytes and oligodendrocytes of aged baboons. J Neuropathol Exp Neurol 59:39–52
Schultz C, Hubbard B, Rub U, Braak E, Braak H (2000b) Age-related progression of tau pathology in brains of baboons. Neurobiol Aging 21:905–911
Sharma M, Gupta Y (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489–2498
Siddique Z, Peters A (1999) The effect of aging on pars compacta of the substantia nigra in rhesus monkeys. J Neuropathol Exp Neurol 58:903–920
Sparks D, Schreurs B (2003) Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. PNAS 100:11065–11069
Sparks D, Friedland R, Petanceska S, Schreurs B, Shi J, Perry G (2006) Trace copper levels in the drinking water, but not zinc or aluminium influence CNS Alzheimer-like pathology. J Nutr Health Aging 10:247–254
Speakman J (2005) Body size, energy metabolism and lifespan. J Exp Biol 208:1717–1730
Spires TL, Hyman BT (2005) Transgenic models of Alzheimer’s disease: learning from animals. NeuroRx 2:423–437
Stieler J, Boerema A, Bullmann T, Kohl F, Strijkstra A (2008) Activity state profile of tau kinases in hibernating animals. In: Lovegrove B, McKechnie A (eds) Hypometabolism in animals: hibernation turpor and cryobiology. University of KwaZulu-Natal, Pietermaritzburg, pp 133–142
Stieler J, Bullmann T, Kohl F, Toien O, Bruckner M, Hartig W, Barnes B, Arendt T (2011) The physiological link between metabolic rate depression and tau phosphorylation in mammalian hibernation. PLoS ONE 6:e14530
Suh YH, Checler F (2002) Amyloid precursor protein, presenilins, and α-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharm Rev 54:469–525
Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa K (2002) Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci 22:133–141
Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima K, Iwasaki K (2002) Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. PNAS 99:13896–13901
Van Dam D, D’Hooge R, Staufenbiel M, van Ginneken C, van Meir F, De Deyn P (2003) Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci 17:388–396
van Groen T, Kadish I, Popović N, Popović M, Caballero-Bleda M, Baño-Otálora B, Vivanco P, Rol MA, Madrid JA (2011) Age-related brain pathology in Octodon degu: blood vessel, white matter and Alzheimer-like pathology. Neurobiol Aging 32(9):1651–1661
Vardy ER, Catto AJ, Hooper NM (2005) Proteolytic mechanisms in amyloid-beta metabolism: therapeutic implications for Alzheimer’s disease. Trends Mol Med 11:464–472
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 286:735–741
Voytko M (1998) Nonhuman primates as models for aging and Alzheimer’s disease. Lab Anim Sci 48:611–617
Walker L (1991) Animal models of cerebral amyloidosis. Bull Clin Neurosci 56:86–96
Walker L (1997) Animal models of cerebral beta-amyloid angiopathy. Brain Res Rev 30:70–84
Walsh D, Klyubin I, Fadeeva J, Cullen W, Anwyl R, Wolfe M, Rowan M, Selkoe D (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539
Weinstock M, Shoham S (2004) Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J Neural Transm 111:347–366
Whiteman I, Gervasio O, Cullen K, Guillemin G, Jeong E, Witting P, Antao S, Minamide L, Bamburg J, Goldsbury C (2009) Activated actin-depolymerising factor/cofilin sequesters phosphorylated microtubule-associated protein during the assembly of Alzheimer-like neuritic cytoskeletal striations. J Neurosci 29:12994–13005
Winton M, Lee E, Sun W, Wong M, Leight S, Zhang B, Trojanowski J, Lee VM-Y (2011) Intraneuronal APP, not free Aβ peptides in 3×Tg-AD mice: Implications for tau versus Aβ-mediated Alzheimer neurodegeneration. J Neurosci 31:7691–7699
Wisniewski HM, Ghetti B, Terry R (1973) Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol 32:566–584
Wong G, Manfra D, Poulet F, Zhang Q, Josien H, Bara T (2004) Chronic treatment with γ-secretase inhibitor LY-411, 575 inhibit β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 279:12876–12882
Woodruff-Pak D, Agelan A, Del Valle L (2007) A rabbit model Alzheimer’s disease: valid at neuropathological, cognitive, and therapeutic levels. J Alzheimers Dis 11:371–383
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Acknowledgments
We thank the Alzheimer’s Association (USA), the University of New South Wales, the Rebecca L. Cooper Medical Foundation, the Perpetual Foundation, the Baxter Foundation, the Mason Foundation, the Curran Foundation, the National Health and Medical Research Council (NHMRC) and Alzheimer Australia for supporting our work. A.G.P.’s and P.M.’s research was partially support by a FIRCA NIH grant to Alfredo Kirkwood 1 R03 TW007171-01 A1 and Iniciativa Cientifica Milenio CINV IC09-022-P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braidy, N., Muñoz, P., Palacios, A.G. et al. Recent rodent models for Alzheimer’s disease: clinical implications and basic research. J Neural Transm 119, 173–195 (2012). https://doi.org/10.1007/s00702-011-0731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0731-5