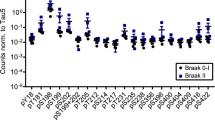
Abstract
Frontotemporal lobar degeneration (FTLD) is a common cause of presenile dementia characterised by behavioural and language disturbances. Pick’s disease (PiD) is a subtype of FTLD, which presents with intraneuronal inclusions consisting of hyperphosphorylated tau protein aggregates. Although Alzheimer’s disease (AD) is also characterised by tau lesions, these are both histologically and biochemically distinct from the tau aggregates found in PiD. What determines the distinct characteristics of these tau lesions is unknown. As phosphorylated, soluble tau has been suggested to be the precursor of tau aggregates, we compared both the level and phosphorylation profile of tau in tissue extracts of AD and PiD brains to determine whether the differences in the tau lesions are reflected by differences in soluble tau. Levels of soluble tau were decreased in AD but not PiD. In addition, soluble tau was phosphorylated to a greater extent in AD than in PiD and displayed a different phosphorylation profile in the two disorders. Consistently, tau kinases were activated to different degrees in AD compared with PiD. Such differences in solubility and phosphorylation may contribute, at least in part, to the formation of distinct tau deposits, but may also have implications for the clinical differences between AD and PiD.




Similar content being viewed by others
References
Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2:783–787
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98:6923–6928
Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672
Bell K, Cairns NJ, Lantos PL, Rossor MN (2000) Immunohistochemistry distinguishes: between Pick’s disease and corticobasal degeneration. J Neurol Neurosurg Psychiatry 69:835–836
Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM (2003) Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 60:1005–1011
Buee L, Delacourte A (1999) Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 9:681–693
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33:95–130
Buee-Scherrer V, Goedert M (2002) Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases in intact cells. FEBS Lett 515:151–154
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, Schneider JA, Tenenholz Grinberg L, Halliday GM et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 114:5–22
Chen F, David D, Ferrari A, Gotz J (2004) Posttranslational modifications of tau—role in human tauopathies and modeling in transgenic animals. Curr Drug Targets 5:503–515
Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, Budka H, Carmona M, Giaccone G, Krebs B et al (2007) Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol 66:35–46
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314:777–781
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Gotz J (2001) Tau and transgenic animal models. Brain Res Brain Res Rev 35:266–286
Gotz J, Ittner LM (2008) Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci 9:532–544
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Hoerndli FJ, Toigo M, Schild A, Gotz J, Day PJ (2004) Reference genes identified in SH-SY5Y cells using custom-made gene arrays with validation by quantitative polymerase chain reaction. Anal Biochem 335:30–41
Ittner LM, Koller D, Muff R, Fischer JA, Born W (2005a) The N-terminal extracellular domain 23–60 of the calcitonin receptor-like receptor in chimeras with the parathyroid hormone receptor mediates association with receptor activity-modifying protein 1. Biochemistry 44:5749–5754
Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F et al (2005b) Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol 4:11
King ME, Ghoshal N, Wall JS, Binder LI, Ksiezak-Reding H (2001) Structural analysis of Pick’s disease-derived and in vitro-assembled tau filaments. Am J Pathol 158:1481–1490
Kril JJ, Halliday GM (2001) Alzheimer’s disease: its diagnosis and pathogenesis. Int Rev Neurobiol 48:167–217
Ledesma MD, Avila J, Correas I (1995) Isolation of a phosphorylated soluble tau fraction from Alzheimer’s disease brain. Neurobiol Aging 16:515–522
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159
Mazanetz MP, Fischer PM (2007) Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov 6:464–479
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, Work Group on Frontotemporal Dementia, Pick’s Disease (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809
Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF (1999) Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58:1010–1019
Pei JJ, Braak H, An WL, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I (2002) Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Brain Res Mol Brain Res 109:45–55
Probst A, Tolnay M, Langui D, Goedert M, Spillantini MG (1996) Pick’s disease: hyperphosphorylated tau protein segregates to the somatoaxonal compartment. Acta Neuropathol 92:588–596
Rizzu P, Joosse M, Ravid R, Hoogeveen A, Kamphorst W, van Swieten JC, Willemsen R, Heutink P (2000) Mutation-dependent aggregation of tau protein and its selective depletion from the soluble fraction in brain of P301L FTDP-17 patients. Hum Mol Genet 9:3075–3082
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–754
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Uchihara T, Ikeda K, Tsuchiya K (2003) Pick body disease and Pick syndrome. Neuropathology 23:318–326
Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM (2007) Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc Natl Acad Sci USA 104:10252–10257
Zhukareva V, Sundarraj S, Mann D, Sjogren M, Blenow K, Clark CM, McKeel DW, Goate A, Lippa CF, Vonsattel J-P et al (2003) Selective reduction of soluble tau proteins in sporadic and familial frontotemporal dementias: an international follow-up study. Acta Neuropathol 105:469–476
Acknowledgments
We thank Dr Vanessa Young for assistance with preparation of the tissue sections. Dr Peter Davies and Dr Peter Seubert generously provided antibodies. Tissues were received from the Australian Brain Donor Programs Prince of Wales Medical Research Institute Tissue Resource Centre, which is supported by the National Health and Medical Research Council of Australia and from the Cambridge Brain Bank. This research was supported by grants to J. Götz. from The University of Sydney, The Medical Foundation (University of Sydney), the National Health and Medical Research Council, Judith Jane Mason & Harold Stannett Williams Memorial Foundation, the Australian Research Council and New South Wales Government through the Ministry for Science and Medical Research (BioFirst Grant), and to L.M. Ittner from the Australian Research Council, the University of Sydney and the Deutsche Forschungsgesellschaft and the National Health and Medical Research Council (JK, LI, JG, GH). J. Götz is a Medical Foundation Fellow and G.M. Halliday is a National Health and Medical Research Council Principal Research Fellow. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Eersel, J., Bi, M., Ke, Y.D. et al. Phosphorylation of soluble tau differs in Pick’s disease and Alzheimer’s disease brains. J Neural Transm 116, 1243–1251 (2009). https://doi.org/10.1007/s00702-009-0293-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0293-y