Abstract
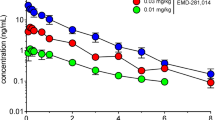
In the present study, we evaluated the effects of subchronic blockade of mGluR5 by 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) on learning, anxiety and levodopa-induced dyskinesia in rats. In addition, we excluded the possibility that subchronic treatment produced pharmacokinetic changes using brain microdialysis. MTEP (5 mg/kg) impaired spatial learning in a radial maze task and contextual fear conditioning (CFC) when administered acutely, and the same effect was observed following a 4-day pre-treatment regime. Similarly, MTEP (5 mg/kg) exerted anxiolytic-like effects in CFC when given before the test whether administered after acute or sub-chronic treatment. Similarly, in levodopa-induced dyskinesia, sub-chronic (7 subsequent days) treatment with MTEP (5 mg/kg) resulted in a significant reduction in abnormal involuntary movements (AIMs), comparable to single acute administration. The data indicate that tolerance does not develop to the anxiolytic and antidyskinetic effects of mGluR5 antagonist MTEP at least at the used treatment mode and tested doses. However, at least at the doses tested, also no tolerance to the memory impairing effect of MTEP was observed. Depending on the indication and model, the amnesic effects of MTEP should be taken into account as a potential limitation, also after repetitive treatment.




Similar content being viewed by others
References
Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA (2003) In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand. Eur J Pharmacol 473:35–40
Andersson M, Hilbertson A, Cenci MA (1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis 6:461–474
Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND (2004) The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1, 3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29:1971–1979
Cenci MA, Lee CS, Bjorklund A (1998) l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706
Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237
De Novellis V, Siniscalco D, Galderisi U, Fuccio C, Nolano M, Santoro L, Cascino A, Roth KA, Rossi F, Maione S (2004) Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology 46:468–479
Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W (2006) Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull 69:318–326
Dekundy A, Lundblad M, Danysz W, Cenci MA (2007) Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179:76–89
Gravius A, Pietraszek M, Schafer D, Schmidt WJ, Danysz W (2005) Effects of mGluR1 and mGluR5 antagonists on negatively reinforced learning. Behav Pharmacol 16:113–121
Gravius A, Barberi C, Schäfer D, Schmidt WJ, Danysz W (2006) The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats—a comparison. Neuropharmacology 51:1146–1155
Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P (2004) Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol 74:271–300
Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM (2003) Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson’s disease. Exp Neurol 183:458–468
Hodgson RA, Lu SX, Bleickardt SJ, Cohen-Williams ME, Jones N, Baptista MA, Werner BJ, Hyde LA, Varty GB (2007) Tolerance to the effects of MTEP in animal models of anxiety-like behavior, cognition, and motor movement. Society for Neuroscience Meeting Abstract, San Diego
Huang D, Poon SF, Chapman DF, Chung J, Cramer M, Reger TS, Roppe JR, Tehrani L, Cosford ND, Smith ND (2004) 2-(2-[3-(Pyridin-3-yloxy)phenyl]-2H-tetrazol-5-yl) pyridine: a highly potent, orally active, metabotropic glutamate subtype 5 (mGlu5) receptor antagonist. Bioorg Med Chem Lett 14:5473–5476
Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA (2004) Transcriptome analysis in a rat model of l-DOPA-induced dyskinesia. Neurobiol Dis 17:219–236
Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F (2008) Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis 29:161–168
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Malberg JK, Sukoff Rizzo SJ, Khawaja X, Rosenzweig-Lipson S (2006) Anxiolytic-like effects of the mGlur5 antagonist MTEP: determination of tolerance after subchronic administration. Society for Neuroscience Meeting Abstract, Atlanta
Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497
Naie K, Manahan-Vaughan D (2004) Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex 14:189–198
Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci 6:501–506
Pietraszek M, Sukhanov I, Maciejak P, Szyndler J, Gravius A, Wislowska A, Plaznik A, Bespalov AY, Danysz W (2005) Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats. Eur J Pharmacol 514:25–34
Pilc A, Klodzinska A, Branski P, Nowak G, Palucha A, Szewczyk B, Tatarczynska E, Chojnacka-Wojcik E, Wieronska JM (2002) Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology 43:181–187
Roppe J, Smith ND, Huang D, Tehrani L, Wang B, Anderson J, Brodkin J, Chung J, Jiang X, King C, Munoz B, Varney MA, Prasit P, Cosford ND (2004) Discovery of novel heteroarylazoles that are metabotropic glutamate subtype 5 receptor antagonists with anxiolytic activity. J Med Chem 47:4645–4648
Samadi P, Gregoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, Belanger N, Meltzer LT, Bedard PJ, Di Paolo T (2008) mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging 29(7):1040–1051. doi:10.1016/j.neurobiolaging.2007.02.005
Sevostianova N, Danysz W (2006) Analgesic effects on mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology 51(3):623–630
Simonyi A, Schachtman TR, Christoffersen GR (2005) The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect 18:353–361
Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C (2000) Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther 295:1267–1275
Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R (2003) Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol 14:257–277
Tel BC, Zeng BY, Cannizzaro C, Pearce RK, Rose S, Jenner P (2002) Alterations in striatal neuropeptide mRNA produced by repeated administration of l-DOPA, ropinirole or bromocriptine correlate with dyskinesia induction in MPTP-treated common marmosets. Neuroscience 115:1047–1058
Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R (2001) Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology 40:1–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gravius, A., Dekundy, A., Nagel, J. et al. Investigation on tolerance development to subchronic blockade of mGluR5 in models of learning, anxiety, and levodopa-induced dyskinesia in rats. J Neural Transm 115, 1609–1619 (2008). https://doi.org/10.1007/s00702-008-0098-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0098-4