Abstract
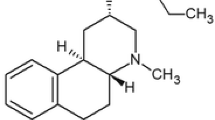
Schizophrenia may reflect a sensitization of dopaminergic (DA) function. Apomorphine (Apo), a DA receptor agonist, induces both sensitization and tolerance of DA function in rodents depending on dose intervals. We investigated sensitization and tolerance to Apo in healthy male volunteers. After a period of acclimatization to the experimental setting (Day 1) subjects were assigned randomly to two groups: Group A subjects received seven injections of placebo (physiological saline) (PLA) and Group B subjects received seven injections of Apo HCl (7 μg/kg sc) under double-blind conditions at 2 h intervals commencing at 0930 hours (Day 2) after an overnight fast. Twelve hours after the seventh injection, i.e. on Day 3, after an overnight fast all subjects received an injection of Apo. Serial samples of blood commencing at 0900 hours were drawn after the first and last injection in both groups for assay of growth hormone (GH), prolactin (PRL) and cortisol by radioimmunoassay; sleepiness was measured using the Analog Sleepiness Rating Scale and yawning recorded by video recorder. The GH response in Group B (N = 8) was (a) decreased after the eighth injection of Apo compared with the first injection of Apo (P = 0.03) and (b) decreased after the eighth injection of Apo compared with the first injection of Apo in Group A (N = 10) (P = 0.001). The number of yawns in Group B was significantly decreased after the eighth injection of Apo compared with the first injection of Apo (P = 0.042). PRL, cortisol and sleepiness were not significantly different between the first and eighth injection of Apo. Sensitization was not observed in any of the measures studied. These results are compatible with induction of acute tolerance of DA-mediated GH and yawning responses. The method used provides a safe pharmacological paradigm to examine plasticity of DA mechanisms in man. Results are discussed in the context of possible therapeutic implications for schizophrenia.



Similar content being viewed by others
References
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 155:761–767
Akiyama K, Kanazaki A, Tsuchida K, Ujike H (1994) Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr Res 12:251–257
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disoders, 4th edn. American Psychiatric Association, Washington, DC
Angrist B (1983) Psychosis induced by central nervous system timulants and related drugs. In: Creese I (ed) Stimulants: neurochemical behavioral and clinical perspectives. Raven Press, New York, pp 1–30
Bedingfield JB, Calder LD, Karler R (1996) Comparative behavioral sensitization to stereotypy by direct and indirect dopamine agonists in CG-1 mice. Psychopharmacology 124:219–225
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 94:2569–2574
Brown GM, Cleghorn JM, Kaplan RD, Szechtman H, Brown PJ, Szechtman B, Mitton J (1988) Longitudinal growth hormone studies in schizophrenia. Psychiatry Res 24:123–126
Butcher LL, Anden NE (1969) Effects of apomorphine and amphetamine on schedule-controlled behavior: reversal of tetrabenazine suppression and dopaminergic correlates. Eur J Pharmacol 6:255–264
Carroll BJ, Curtis GC, Kokmen E (1977) Paradoxical response to dopamine agonists in tardive dyskinesia. Am J Psychiatry 134:785–789
Carskadon MA, Dement WC (1981) Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology 18:107–113
Castro R, Abreu P, Calzadilla CH, Rodriguez M (1985) Increased or decreased locomotor response in rats following repeated administration of apomorphine depends on dosage interval. Psychopharmacology 85:333–339
Colzi A, Turner K, Lees AJ (1998) Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 64:573–576
Corsini GU, Del Zompo M, Manconi S, Cianchetti C, Mangoni A, Gessa GL (1977) Sedative, hypnotic and antipsychotic effects of low doses of apomorphine in man. Adv Biochem Psychopharmacol 16:645–648
Corsini GU, Piccardi MP, Bochetta A, Bernardi F, Del Zompo M (1981) Behavioural effects of apomorphine in man: dopamine receptor implications. In: Corsini GU, Gessa GL (eds) Apomorphine and other dopaminomimetics. Clinical Pharmacology, vol 2. Raven Press, New York, pp 13–24
Davis KL, Kahn RS, Ko G, Davidson M (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486
Dépatie L, Lal S (2001) Apomorphine and the dopamine hypothesis of schizophrenia—a dilemma? J Psychiatr Neurosci 26:203–220
Deschaies P, Bedad P, Falardeau P, DiPaolo T (1984) Behavioural and biochemical evidence of apomorphine-induced supersensitivity of the striatal dopamine receptors. Neuropharmacology 23:1219–1222
Druhan JP, Jakob A, Stewart J (1993) The development of behavioral sensitization to apomorphine is blocked by MK-801. Eur J Pharmacol 243:73–77
Feldman F, Susselman S, Barrera SE (1945) A note on apomorphine as a sedative. Am J Psychiatry 102:403–405
First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for DSM-IV axis I disorders. New York State Psychiatric Institute, Biometrics Research Department, New York
First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L (1997) Structured clinical interview for DSM IV personality disorders (SCID-II). New York State Psychiatric Institute, Biometrics Research Dept, New York
Gancher ST, Woodward WR, Boucher B, Nutt JG (1989) Peripheral pharmacokinetics of apomorphine in humans. Ann Neurol 26:232–238
Gancher ST, Nutt JG, Woodward WR (1992) Time course of tolerance to apomorphine in parkinsonism. Clin Pharmacol Ther 52:504–510
Gancher ST, Nutt JG, Woodward WR (1995) Apomorphine infusional therapy in Parkinson’s disease: clinical utility and lack of tolerance. Mov Disord 10:37–43
Gancher S, Mayer A, Youngman S (1996a) Changes in apomorphine pharmacodynamics following repeated treatment in 6-hydroxydopamine-lesioned rats. Brain Res 729:190–196
Gancher ST, Woodward WR, Nutt JG (1996b) Apomorphine tolerance in Parkinson’s disease: lack of a dose effect. Clin Neuropharmacol 19:59–64
Glenthoj BY (1995) The brain dopaminergic system. Pharmacological behavioural and electrophysiological studies. Dan Med Bull 42:1–21
Glenthoj B, Mogensen J, Laursen H, Holm S, Hemmingsen R (1993) Electrical sensitization of the meso-limbic dopaminergic system in rats: a pathogenic model for schizophrenia. Brain Res 619:39–54
Grandas F, Obeso JA (1989) Motor response following repeated apomorphine administration is reduced in Parkinson’s disease. Clin Neuropharmacol 12:14–22
Grandas F, Gancher S, Lera G, Rodriguez M, Woodward WR, Nutt J, Obeso JA (1992) Time interval between repeated injections conditions the duration of motor improvement to apomorphine in Parkinson’s disease. Neurology 42:1287–1290
Hughes AJ, Bishop S, Kleedorfer B, Turjanski N, Fernandez W, Lees AJ, Stern GM (1993) Subcutaneous apomorphine in Parkinson’s disease: response to chronic administration for up to five years. Mov Disord 8:165–170
Isaacs B, MacArthur JG (1954) Influence of chlorpromazine and promethazine on vomiting induced with apomorphine in man. Lancet 267:570–572
Kaňovský P, Kubova D, Bares M, Hortova H, Streitova H, Rektor I, Znojil V (2002) Levodopa-induced dyskinesias and continuous subcutaneous infusions of apomorphine: results of a two-year, prospective follow-up. Mov Disord 17:188–191
Kapur S, Mamo D (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27:1081–1090
Kapur S, Remington G (2001) Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry 50:873–883
Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, Watt H, Bhatia K, Quinn N, Lees AJ (2005) Continuous subcutaneous apomorphine therapy improves dyskinesia in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord 20:151–157
Kaul PN, Conway MW (1971) Induction and inhibition of in vivo glucuronidation of apomorphine in mice. J Pharm Sci 60:93–95
Lal S (1987) Growth hormone and schizophrenia. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven Press, New York, pp 809–818
Lal S (1988) Apomorphine in the evaluation of dopaminergic function in man. Prog Neuropsychopharmacol Biol Psychiatry 12:117–164
Lal S, de la Vega CE (1975) Apomorphine and psychopathology. J Neurol Neurosurg Psychiatry 38:722–726
Lal S, Sourkes TL, Missala K, Belendiuk G (1972a) Effects of apomorphine and emetine alkaloids on central dopaminergic mechanisms in rats. Eur J Pharmacol 20:71–79
Lal S, de la Vega CE, Sourkes TL, Friesen HG (1972b) Effect of apomorphine on human growth hormone secretion. Lancet 2:661
Lal S, De la Vega CE, Sourkes TL, Friesen HG (1973) Effect of apomorphine on growth hormone, prolactin, luteinizing hormone and follicle-stimulating hormone levels in human serum. J Clin Endocrinol Metab 37:719–724
Lal S, Grassino A, Thavundayil JX, Dubrovsky B (1987) A simple method for the study of yawning in man induced by the dopamine receptor agonist, apomorphine. Prog Neuropsychopharmacol Biol Psychiatry 11:223–228
Lal S, Tesfaye Y, Thavundayil JX, Thompson TR, Kiely ME, Nair NPV, Grassino A, Dubrovsky B (1989) Apomorphine: clinical studies on erectile impotence and yawning. Prog Neuropsychopharmacol Biol Psychiatry 13:329–339
Lieberman JA, Kane JM, Alvir J (1987) Provocative tests with psychostimulant drugs in schziophrenia. Psychopharmaoclogy 91:415–433
Lieberman JA, Kinon BJ, Loebe AD (1990) Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull 16:97–110
Lieberman JA, Sheitman BB, Kinon BJ (1997) Neurochemical sensitization in the pathophysiology of schizohrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 17:205–229
Martin JB, Lal S, Tolis G, Friesen HG (1974) Inhibition by apomorphine of prolactin secretion in patients with elevated serum prolactin. J Clin Endocrinol Metab 39:180–182
Mattingly BA, Gotsick JE, Marin C (1989) Locomotor activity and stereotypy in rats following repeated apomorphine treatments at 1-, 3- or 7-day intervals. Pharm Biochem Behav 31:871–875
Mattingly BA, Rowlett JK, Graff JT, Hatton BJ (1991) Effects of selective D1 and D2 dopamine antagonists on the development of behavioral sensitization to apomorphine. Psychopharmacology 105:501–507
Melis MR, Argiolas A, Gessa GL (1987) Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res 415:98–104
Müller-Spahn F, Modell S, Ackenheil M, Brachner A, Kurtz G (1998) Elevated response of growth hormone to graded doses of apomorphine in schizophrenic patients. J Psychiatr Res 32:265–271
Nair NPV, Lal S, Iskandar HI, Etienne P, Wood PL, Guyda H (1982) Effect of sulpiride, an atypical neuroleptic, on apomorphine-induced growth hormone secretion. Brain Res Bull 8:587–591
Pandey GN, Garver DL, Tamminga C, Erickson S, Ali SI, Davis JM (1977) Post- synaptic supersensitivity in schizophrenia. Am J Psychiatry 134:518–522
Sato M (1979) Experimental study of onset and relapsing mechanisms of chronic methamphetamine psychosis. Psychiatr Neurol Jpn 81:21–32
Sato M, Chen CC, Akiyama K, Otsuki S (1983) Acute exacerbation of paranoid psychotic state after longterm abstinence in patients with previous methamphetamine psychosis. Biol Psychiatry 18:429–440
Sato M, Numachi Y, Hamamura T (1992) Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr Bull 18:115–122
Seeman P, Van Tol HHM (1994) Dopamine receptor pharmacology. TIPS 15:264–270
Seeman P, Weinshenker D, Quirion R, Srivastava L, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundy M, O’Dowd BF, George SR, Perreault ML, Mänistö PT, Robinson S, Palmiter RD, Tallerico T (2005) Dopamine supersensitivity correlates with D2high states, implying many paths to psychosis. Proc Natl Acad Sci 102:3513–3518
Segal DS, Schuckit MA (1983) Animal models of stimulant induced psychosis. In: Creese I (ed) Stimulants: neurochemical, behavioral and clinical perspectives. Raven Press, New York, pp 131–167
Serra G, Collu M, Gessa GL (1987) Yawning is elicited by D2 dopamine agonists but is blocked by the D1 antagonist, SCH 23390. Psychopharmacology 91:330–333
Smith RC, Tamminga C, Davis JM (1977) Effect of apomorphine on schizophrenic symptoms. J Neural Transm 40:171–176
Szechtman H, Cleghorn JM, Brown GM, Kaplan RD, Franco S, Rosenthal K (1988) Sensitization and tolerance to apomorphine in men: yawning, growth hormone, nausea and hyperthermia. Psychiatry Res 23:245–255
Tamminga CA, Gotts MD, Thaker GK, Alphs LD, Foster NL (1986) Dopamine agonist treatment of schizophrenia with N-propylnorapomorphine. Arch Gen Psychiatry 43:398–402
Tamminga CA, Schaffer MH, Smith RC, Davis JM (1978) Schizophrenic symptoms improve with apomorphine. Science 200:567–568
Tsang D, Lal S (1977) Effect of monoamine receptor agonists and antagonists on cyclic AMP accumulation in human cerebral cortex slices. Can J Physiol Pharmacol 55:1263–1269
Winkler JD, Weiss B (1986) Reversal of supersensitive apomorphine-induced rotational behavior in mice by continuous exposure to apomorphine. J Pharmacol Exp Ther 238:242–247
Acknowledgments
This study received support from the Medical Research Council of Canada (now called the Canadian Institutes of Health Research to S.L. We thank Norbert Schmitz PhD for statistical advice and Nadia Zajac for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lal, S., Thavundayil, J.X., Ng Ying Kin, N.M.K. et al. Induction of tolerance of dopaminergic responses in man. J Neural Transm 115, 1189–1198 (2008). https://doi.org/10.1007/s00702-008-0068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0068-x