Abstract
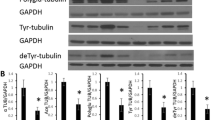
CacyBP/SIP was originally identified as an S100A6 (calcyclin) target and later on as a Siah-1 interacting protein. Recently, we have shown that CacyBP/SIP interacts with tubulin, which suggests its involvement in the reorganization of microtubules. In this work we examined the localization of CacyBP/SIP in cultured neurons and in brain neurons of young and aged rats, and compared this localization with that of tubulin and the tau protein. We have found that in neurons of young rats CacyBP/SIP, tubulin and tau are present in the cytoplasm and in the neuronal processes, whereas in aged animals CacyBP/SIP and tau are mainly seen in the cytoplasm of the neuronal somata. In aged rats, these changes are also accompanied by a different localization pattern of tubulin. Thus, our results show that localization of CacyBP/SIP in brain neurons is similar to that observed for tau and tubulin, which points to the involvement of CacyBP/SIP in cytoskeletal physiology.





Similar content being viewed by others
References
Alonso AD, Grundke-Iqbal I, Barra HS et al (1997) Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA 94:298–303
Alonso AD, Zaidi T, Novak M et al (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98:6923–6928
Alonso Adel C, Li B, Grundke-Iqbal I et al (2006) Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA 103:8864–8869
Andreadis A (2005) Tau gene alternative splicing: expression pattern, regulation and modulation of unction in normal brain and neurodegenerative diseases. Biochim Biophys Acta 1739:91–103
Au KW, Kou CY, Woo AY et al (2006) Calcyclin binding protein promotes DNA synthesis and differentiation in rat neonatal cardiomyocytes. J Cell Biochem 98:555–566
Avila J, Lucas JJ, Perez M et al (2004) Role of Tau in both physiological and pathological conditions. Physiol Rev 84:361–384
Baas PW, Qiang L (2005) Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biol 15:183–187
Bendiske J, Caba E, Brown QB et al (2002) Intracellular deposition, microtubule destabilization, and transport failure: an ‘early’ pathogenic cascade leading to synaptic decline. J Neuropathol Exp Neurol 61:640–650
Brandt R, Hundelt M, Shahani N (2005) Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Bichim Biophys Acta 1739:331–354
Buée L, Bussiere T, Buee-Scherrer V et al (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev 33:95–130
Charriere-Bertrand C, Nunez J (1992) Regulation of tubulin, Tau and microtubule associated protein 2 expression during mouse brain development. Neurochem Int 21:535–541
Dehmelt L, Halpain S (2005) The MAP2/Tau family of microtubule-associated proteins. Genome Biol 6:204–207
Desai A, Mitchison TJ (1997) Microtubule polymerisation dynamics. Ann Rev Cell Dev Biol 13:83–117
Drechsel DN, Hyman AA, Cobb MH et al (1992) Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell 3:1141–1154
Elder GA, Friedrich VL, Margita A et al (1999) Age-related atrophy of motor axons in mice deficient in the mid-sized neurofilament subunit. J Cell Biol 146:181–192
Feinstein SC, Wilson L (2005) Inability of tau to properly regulate neuronal microtubule dynamics: a loss-of-function mechanism by which tau might mediate neuronal cell death. Biochim Biophys Acta 1739:268–279
Figiel I, Dzwonek K (2007) TNF α and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res 1131:17–28
Filipek A, Jastrzebska B, Nowotny M et al (2002a) CacyBP/SIP, a calcyclin and Siah-1-interacting protein, binds EF-hand proteins of the S100 family. J. Biol Chem 277:28848–28852
Filipek A, Jastrzebska B, Nowotny M et al (2002b) Ca2+-dependent translocation of the calcyclin-binding protein in neurons and neuroblastoma NB-2a cells. J Biol Chem 277:21103–21109
Filipek A, Kuznicki J (1998) Molecular cloning and expression of a mouse brain cDNA encoding a novel protein target of calcyclin. J Neurochem 70:1793–1798
Filipek A, Puzianowska M, Cieślak B et al (1993) Calcyclin-Ca2+-binding protein homologous to glial S-100 beta is present in neurones. Neuroreport 4:383–386
Filipek A, Wojda U (1996) p30, a novel protein target of mouse calcyclin (S100A6). Biochem J 320:585–587
Fukushima T, Zapata JM, Singha NC et al (2006) Critical function for SIP, a ubiquitin E3 ligase component of the beta-catenin degradation pathway, for thymocyte development and G1 checkpoint. Immunity 24:29–39
Gundersen GG, Cook TA (1999) Microtubules and signal transduction. Curr Opin Cell Biol 11:81–94
Herington JL, Bi J, Martin JD et al (2007) Beta-catenin (CTNNB1) in the mouse uterus during decidualization and the potential role of two pathways in regulating its degradation. J Histochem Cytochem. 55:963–74
Hirokawa N, Takemura R (2003) Biochemical and molecular characterization of diseases linked to motor proteins. Trends Biochem Sci 28:558–565
Howard J, Hyman AA (2007) Microtubule polymerases and depolymerases. Curr Opin Cell Biol 19:31–35
Iqbal K, Grundke-Iqbal I, Zaidi T et al (1986) Are Alzheimer neurofibrillary tangles insoluble polymers? Life Sci 38:1695–1700
Jastrzebska B, Filipek A, Nowicka D et al (2000) Calcyclin (S100A6) binding protein (CacyBP) is highly expressed in brain neurons. J Histochem Cytochem 48:1195–1202
Kinoshita K, Noetzel TL, Arnal I et al (2006) Global and local control of microtubule destabilization promoted by a catastrophe kinesin MCAK/XKCM1. J Muscle Res Cell Motil 27:107–114
Köpke E, Tung YC, Shaikh S et al (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268:24374–24384
Kosik KS, Shimura H (2005) Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta 1739:298–310
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lovestone S, McLoughlin DM (2002) Protein aggregates and dementia: is there a common toxicity? J Neurol Neurosur Psych 72:152–161
Mandelkow E, Mandelkow EM (1998) Tau in Alzheimer’s disease. Trends Cell Biol 8:425–427
Mandelkow E, von Bergen M, Biernat J et al (2007) Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Patholol 17:83–90
Matsuzawa S, Reed JC (2001) Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell 7:915–926
Niewiadomska G, Baksalerska-Pazera M (2003) Age-dependent changes in axonal transport and cellular distribution of Tau1 in the rat basal forebrain neurons. NeuroRep 14:1701–1706
Niewiadomska G, Baksalerska-Pazera M, Lenarcik I (2005) Breakdown in retrograde axonal transport and altered cellular distribution of phospho-Tau proteins during aging. Annals New York Acad Sci 1048:287–295
Niewiadomska G, Baksalerska-Pazera M, Riedel G (2006a) Cytoskeletal transport in the aging brain: focus on the cholinergic system. Rev Neurosci 17:581–618
Niewiadomska G, Baksalerska-Pazera M, Lenarcik I et al (2006b) Compartmental protein expression of Tau, GSK-3β and TrkA in cholinergic neurons of aged rats. J Neural Transm 113:1733–1746
Nowotny M, Spiechowicz M, Jastrzebska B et al (2003) Calcium-regulated interaction of Sgt1 with S100A6 (calcyclin) and other S100 proteins. J Biol Chem 278:26923–26928
Pircher TJ, Geiger JN, Zhang D et al (2001) Integrative signaling by minimal erythropoietin receptor forms and c-Kit. J Biol Chem 276:8995–9002
Schneider G, Nieznanski K, Kilanczyk E et al (2007) CacyBP/SIP interacts with tubulin in neuroblastoma NB2a cells and induces formation of globular tubulin assemblies. Biochim Biophys Acta 1773:1628–1636
Schuyler SC, Pellman D (2001) Microtubule “plus-end-tracking proteins”: The end is just the beginning. Cell 105:421–424
Silva R, de Farrer M (2002) Tau neurotoxicity without the lesions: a fly challenges a tangled web. Trends Neurosci 25:327–329
Smith DS, Niethammer M, Ayala R et al (2000) Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian LIS1. Nat Cell Biol 2:767–775
Spiechowicz M, Filipek A (2005) The expression and function of Sgt1 protein in eukaryotic cells. Acta Neurobiol Exp 65:161–165
Yang W, Ang LC, Strong MJ (2005) Tau protein aggregation in the frontal and entorhinal cortices as a function of aging. Dev Brain Res 156:127–138
Yang YJ, Liu WM, Zhou JX et al (2006) Expression and hormonal regulation of calcyclin-binding protein (CacyBP) in the mouse uterus during early pregnancy. Life Sci 78:753–60
Wang JZ, Grundke-Iqbal I, Iqbal K (1996) Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res 38:200–208
Wu J, Tan X, Peng X et al (2003) Translocation and phosphorylation of calcyclin binding protein during retinoic acid-induced neuronal differentiation of neuroblastoma SH-SY5Y cells. J Biochem Mol Biol 36:354–358
Acknowledgments
We thank Drs. G. Riedel and W. Leśniak for their critical reading of the manuscript. We also thank Dr. L. Sidorik for polyclonal anti-Sgt1 antibody. This work was supported by grants from the Ministry of Science and Higher Education of Poland to A. Filipek (2 P04A 01030) and G. Niewiadomska (2 PO5A 121), and by statutory funds from the Nencki Institute of Experimental Biology. G. Schneider is a recipient of a scholarship from the President of the Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
702_2008_62_MOESM1_ESM.ppt
Specificity of the anti-Sgt1 antibody estimated by Western blotting. Rat brain extract (40 μg) prepared as described in Material and Methods, paragraph entitled “Tissue extracts preparation, SDS-PAGE and Western blot analysis” was loaded on the 10% SDS polyacrylamide gel, proteins were then blotted into nitrocellulose and incubated with anti-Sgt1 antibody. Then the blots were allowed to react with secondary antibodies conjugated to horseradish peroxidase (Sigma) and developed with ECL chemiluminescence kit (Amersham Biosciences) followed by exposition against an X-ray film. The positions of low molecular mass standard (BioRad) are indicated by arrows. (PPT 72 kb)
Rights and permissions
About this article
Cite this article
Filipek, A., Schneider, G., Mietelska, A. et al. Age-dependent changes in neuronal distribution of CacyBP/SIP: comparison to tubulin and the tau protein. J Neural Transm 115, 1257–1264 (2008). https://doi.org/10.1007/s00702-008-0062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0062-3