Abstract
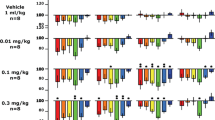
Neonatal exposure of rats to bisphenol-A, an endocrine disruptor, has recently been proposed as a possible animal model of attention-deficit hyperactivity disorder (ADHD), because such rats exhibit motor hyperactivity. To strengthen the face validity of this animal model, the present study replicated the original experiments and additionally analysed both changes in habituation to a novel environment and behavioural responses to methylphenidate, the two phenomena known to be altered in ADHD. Single intracisternal administration of bisphenol-A (20 and 40 μg) into 5-day-old male Wistar rats impaired habituation to a novel environment in the light, but not the dark, phase at 4 weeks of age. Thus, habituation as assessed by time-dependent decrease of locomotor activity, rearing, sniffing and grooming was significantly reduced in bisphenol-A-pretreated rats. Methylphenidate (1 and 3 mg/kg, i.p.) dose-dependently enhanced locomotor activity in both vehicle-pretreated and bisphenol-A-pretreated rats during both the dark and the light phases. Thus, the effects of methylphenidate did not differ between bisphenol-A-pretreated and vehicle-pretreated rats. Apart from a slight methylphenidate-induced increase in rearing and sniffing in bisphenol-A (20 μg)-pretreated rats, the overall effects of methylphenidate on rearing, sniffing and grooming were similar in both vehicle- and bisphenol-A-pretreated rats. It is concluded that neonatal exposure of rats to bisphenol-A is an animal model with limited face validity for ADHD, because the motor hyperactivity and reduced habituation to a novel environment are not accompanied by altered responses to methylphenidate.




Similar content being viewed by others
References
Anckarsäter H, Stahlberg O, Larson T, Hakansson C, Jutblad SB, Niklasson L, Nydén A, Wentz E, Westergren S, Cloninger CR, Gillberg C, Rastam M (2006) The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. Am J Psychiatry 163:1239–1244
Cools AR, Rots NY, Ellenbroek B, de Kloet ER (1993) Bimodal shape of individual variation in behaviour of Wistar rats: the overall outcome of a fundamentally different make-up and reactivity of the brain, the endocrinological and immunological system. Neuropsychobiology 28:100–105
Davids E, Zhang K, Tarazi FI, Baldessarini RJ (2002) Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology 160:92–98
Degen SB, Verheij MM, Cools AR (2004) Genetic background, nature of event, and time of exposure to event direct the phenotypic expression of a particular genotype. Behav Brain Res 154:107–112
Diana M, Collu M, Mura A, Gessa GL (1992) Haloperidol-induced vacuous chewing in rats: suppression by alpha-methyl-tyrosine. Eur J Pharmacol 211:415–419
Dresel S, Krause J, Krause KH, LaFougere C, Brinkbäumer K, Kung HF, Hahn K, Tatsch K (2000) Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med 27:1518–1524
DuPaul GJ, Rapport MD, Vyse SA (1988) ADDH and methylphenidate responders: effects on behavior controlled by complex reinforcement schedules. Int Clin Psychopharmacol 3:349–361
Findling RL, Dogin JW (1998) Psychopharmacology of ADHD: children and adolescents. J Clin Psychiatry 59(Suppl 7):42–49
Fujita S, Okutsu H, Yamaguchi H, Nakamura S, Adachi K, Saigusa T, Koshikawa N (2003) Altered pre- and postsynaptic dopamine receptor functions in spontaneously hypertensive rat: an animal model of attention-deficit hyperactivity disorder. J Oral Sci 45:75–83
Fujita S, Adachi K, Lee J, Uchida T, Koshikawa N, Cools AR (2004) Decreased postsynaptic dopaminergic and cholinergic functions in the ventrolateral striatum of spontaneously hypertensive rat. Eur J Pharmacol 484:75–82
Fuller RW, Hemrick-Luecke SK, Wong DT, Pearson D, Threlkeld PG, Hynes MD III (1983) Altered behavioral response to a D2 agonist, LY141865, in spontaneously hypertensive rats exhibiting biochemical and endocrine responses similar to those in normotensive rats. J Pharmacol Exp Ther 227:354–359
Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401
Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S; American Academy of Child, Adolescent Psychiatry (2002) Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 41(Suppl 2):26S–49S
Greydanus DE, Pratt HD, Patel DR (2007) Attention deficit hyperactivity disorder across the lifespan: the child, adolescent, and adult. Dis Mon 53:70–131
Hynes MD, Langer DH, Hymson DL, Pearson DV, Fuller RW (1985) Differential effects of selected dopaminergic agents on locomotor activity in normotensive and spontaneously hypertensive rats. Pharmacol Biochem Behav 23:445–448
Ikeda H, Adachi K, Hasegawa M, Sato M, Hirose N, Koshikawa N, Cools AR (1999) Effects of chronic haloperidol and clozapine on vacuous chewing and dopamine-mediated jaw movements in rats: evaluation of a revised animal model of tardive dyskinesia. J Neural Transm 106:1205–1216
Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M (2004) Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res 76:423–33
Ishido M, Morita M, Oka S, Masuo Y (2005) Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul Pept 126:145–153
Jansiewicz EM, Newschaffer CJ, Denckla MB, Mostofsky SH (2004) Impaired habituation in children with attention deficit hyperactivity disorder. Cogn Behav Neurol 17:1–8
Koshikawa N, Aoki S, Tomiyama K, Maruyama Y, Kobayashi M (1987) Sulpiride injection into the dorsal striatum increases methamphetamine-induced gnawing in rats. Eur J Pharmacol 133:119–125
Luthman J, Bassen M, Fredriksson A, Archer T (1997) Functional changes induced by neonatal cerebral 6-hydroxydopamine treatment: effects of dose levels on behavioral parameters. Behav Brain Res 82:213–221
Masuo Y, Ishido M, Morita M, Oka S (2004a) Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast 11:59–76
Masuo Y, Morita M, Oka S, Ishido M (2004b) Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept 123:225–234
McCarty R, Kirby RF (1982) Spontaneous hypertension and open-field behavior. Behav Neural Biol 34:450–452
Moy SS (1995) Impaired acquisition and operant responding after neonatal dopamine depletion in rats. Pharmacol Biochem Behav 52:433–441
Myers MM, Musty RE, Hendley ED (1982) Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol 34:42–54
Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD (2001) Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics 107:E105
Sagvolden T, Hendley ED, Knardahl S (1992) Behavior of hypertensive and hyperactive rat strains: hyperactivity is not unitarily determined. Physiol Behav 52:49–57
Sagvolden T, Pettersen MB, Larsen MC (1993) Spontaneously hypertensive rat (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav 54:1047–1055
Sagvolden T (2000) Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24:31–39
Suzuki T, Mizuo K, Nakazawa H, Funae Y, Fushiki S, Fukushima S, Shirai T, Narita M (2003) Prenatal and neonatal exposure to bisphenol-A enhances the central dopamine D1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neuroscience 117:639–644
Swanson JM, McBurnett K, Christian DL, Wigal T (1995) Stimulant medication and treatment of children with ADHD. In: Ollendick TH, Prinz RJ (eds) Advances in clinical child psychology. New York, Plenum Press, pp 265–322
Tsai CF, Lin MT (1988) Locomotor hyperactivity in hypertensive rats. Pharmacology 36:27–34
Van den Buuse M, Veldhuis HD, De Boer S, Versteeg DHG, De Jong W (1986) Central 6-OHDA affects both open-field exploratory behaviour and the development of hypertension in SHR. Pharmacol Biochem Behav 24:15–21
Van den Buuse M, De Jong W (1987) Grooming behavior of spontaneously hypertensive rats. Neurosci Lett 77:71–75
Van den Buuse M, De Jong W (1989) Differential effects of dopaminergic drugs on open-field behavior of spontaneously hypertensive rats and normotensive Wistar-Kyoto rats. J Pharmacol Exp Ther 248:1189–1196
van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA (2007) Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Res 151:211–220
Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ (2002) Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol 12:557–566
Wender PH (1998) Pharmacotherapy of attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry 59(Suppl 7):76–79
Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R (2001) Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci USA 98:1982–1987
Acknowledgments
We thank Dr. Yoshinori Masuo for his technical guidance regarding intracisternal injections of bisphenol-A to pups. These studies were supported by the Sato Fund (NK), Uemura Fund (NK) and Dental Research Centre (HO, NK) at Nihon University School of Dentistry; the Promotion and Mutual Aid Corporation for Private Schools of Japan (MK, NK); and a “Kakenhi” for Young Scientists (B, # 17791332 to SF) and a grant for promotion of multidisciplinary research projects entitled “Translational Research Network on Orofacial Neurological Disorders” (NK, ARC) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiguchi, M., Fujita, S., Oki, H. et al. Behavioural characterisation of rats exposed neonatally to bisphenol-A: responses to a novel environment and to methylphenidate challenge in a putative model of attention-deficit hyperactivity disorder. J Neural Transm 115, 1079–1085 (2008). https://doi.org/10.1007/s00702-008-0044-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0044-5