Summary
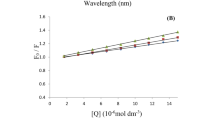
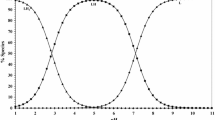
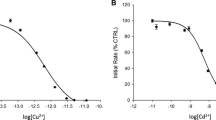
We and others have observed that substrates for copper-containing amine oxidases cause substrate inhibition at high concentrations. Through use of a novel “pseudoquantitative” rapid equilibrium approach, kinetic analyses with human and bovine enzymes indicate that these effects are consistent with substrates binding to oxidised and reduced enzyme forms. Small cations compete with binding of substrates to oxidised and reduced enzyme, influencing both substrate turnover and substrate inhibition patterns. Cations reduce affinity of the resting bovine enzyme for spermidine, but not benzylamine, indicating that the predominant effect of cations on substrate oxidation results from binding to an anionic site outside the active site. However, binding of cations to the active site of the reduced form of both enzymes attenuates substrate inhibition with both spermidine and benzylamine. Our observations have significant practical implications for researchers assaying kinetic behaviour of these enzymes, and particularly those developing novel inhibitors of human copper-containing amine oxidases.
Similar content being viewed by others
References
TT Airenne Y Nymalm H Kidron DJ Smith M Pihlavisto M Salmi S Jalkanen MS Johnson TA Salminen (2005) ArticleTitleCrystal structure of the human vascular adhesion protein-1: Unique structural features with functional implications Protein Sci 14 1964–1974 10.1110/ps.051438105 Occurrence Handle10.1110/ps.051438105 Occurrence Handle1:CAS:528:DC%2BD2MXntVejsbc%3D Occurrence Handle16046623
E Dalfó M Hernandez JM Lizcano KF Tipton M Unzeta (2003) ArticleTitleActivation of human lung semicarbazide-sensitive amine oxidase by a low molecular weight component present in human plasma Biochim Biophys Acta 1638 278–286 Occurrence Handle12878330
ML Di Paolo M Scarpa A Corazza R Stevanato A Rigo (2002) ArticleTitleBinding of cations of group Ia and IIa to bovine serum amine oxidase: Effect on the activity Biophys J 83 2231–2239 10.1016/S0006-3495(02)73983-0 Occurrence Handle10.1016/S0006-3495(02)73983-0 Occurrence Handle1:CAS:528:DC%2BD38XnslSrt78%3D Occurrence Handle12324440
ML Di Paolo R Stevanato A Corazza F Vianello L Lunelli M Scarpa A Rigo (2003) ArticleTitleElectrostatic compared with hydrophobic interactions between bovine serum amine oxidase and its substrates Biochem J 371 549–556 10.1042/BJ20021055 Occurrence Handle10.1042/BJ20021055 Occurrence Handle1:CAS:528:DC%2BD3sXisleqs7k%3D Occurrence Handle12529179
ML Di Paolo M Lunelli M Scarpa A Rigo (2004) ArticleTitlePhosphonium compounds as new and specific inhibitors of bovine serum amine oxidase Biochem J 384 551–558 10.1042/BJ20031883 Occurrence Handle10.1042/BJ20031883 Occurrence Handle1:CAS:528:DC%2BD2cXhtVGku7%2FJ Occurrence Handle15320876
J Elliott BA Callingham D Sharman (1989) ArticleTitleSemicarbazide-sensitive amine oxidase (SSAO) of the rat aorta. Interactions with some naturally occurring amines and their structural analogues Biochem Pharmacol 38 1507–1515 10.1016/0006-2952(89)90191-3 Occurrence Handle10.1016/0006-2952(89)90191-3 Occurrence Handle1:CAS:528:DyaL1MXkvFalu7o%3D Occurrence Handle2719723
I Frébort H Toyama K Matsushita O Adachi (1995) ArticleTitleHalf-site reactivity with p-nitrophenylhydrazine and subunit separation of the dimeric copper-containing amine oxidase from Aspergillus niger Biochem Mol Biol Int 36 1207–1216 Occurrence Handle8535292
A Holt MM Palcic (2006) ArticleTitleA peroxidase-coupled continuous absorbance plate-reader assay for flavin monoamine oxidases, copper-containing amine oxidases and related enzymes Nat Protoc 1 2498–2505 10.1038/nprot.2006.402 Occurrence Handle10.1038/nprot.2006.402 Occurrence Handle1:CAS:528:DC%2BD2sXhtFGjtLrJ Occurrence Handle17406497
A Holt DF Sharman GB Baker MM Palcic (1997) ArticleTitleA continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates Anal Biochem 244 384–392 10.1006/abio.1996.9911 Occurrence Handle10.1006/abio.1996.9911 Occurrence Handle1:CAS:528:DyaK2sXmvVSqtg%3D%3D Occurrence Handle9025956
A Holt B Wieland GB Baker (2004) ArticleTitleAllosteric modulation of semicarbazide-sensitive amine oxidase activities in vitro by imidazoline receptor ligands Br J Pharmacol 143 495–507 10.1038/sj.bjp.0705986 Occurrence Handle10.1038/sj.bjp.0705986 Occurrence Handle1:CAS:528:DC%2BD2cXpsFCns70%3D Occurrence Handle15451775
Hubálek F, Binda C, Khalil A, Li M, Mattevi A, Castagnoli N, Edmondson DE (2005) Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J Biol Chem
G Ignesti (2003) ArticleTitleEquations of substrate-inhibition kinetics applied to pig kidney diamine oxidase (DAO, E.C. 1.4.3.6) J Enzyme Inhib Med Chem 18 463–473 10.1080/14756360310001605543 Occurrence Handle10.1080/14756360310001605543 Occurrence Handle1:CAS:528:DC%2BD2cXpsVei Occurrence Handle15008510
ID Kelly PF Knowles KD Yadav WG Bardsley P Leff RD Waight (1981) ArticleTitleSteady-state kinetic studies on benzylamine oxidase from pig plasma Eur J Biochem 114 133–138 Occurrence Handle1:CAS:528:DyaL3MXpvVOgug%3D%3D Occurrence Handle7215347 Occurrence Handle10.1111/j.1432-1033.1981.tb06183.x
JM Lizcano KF Tipton M Unzeta (2000) ArticleTitleTime-dependent activation of the semicarbazide-sensitive amine oxidase (SSAO) from ox lung microsomes Biochem J 351 789–794 10.1042/0264-6021:3510789 Occurrence Handle10.1042/0264-6021:3510789 Occurrence Handle1:CAS:528:DC%2BD3cXotF2qsLs%3D Occurrence Handle11042135
M Lunelli ML Di Paolo M Biadene V Calderone R Battistutta M Scarpa A Rigo G Zanotti (2005) ArticleTitleCrystal structure of amine oxidase from bovine serum J Mol Biol 346 991–1004 10.1016/j.jmb.2004.12.038 Occurrence Handle10.1016/j.jmb.2004.12.038 Occurrence Handle1:CAS:528:DC%2BD2MXhtFGrsbg%3D Occurrence Handle15701511
CM McEwen SuffixJr (1965) ArticleTitleHuman plasma monoamine oxidase. \(\widetilde{{\rm II}}\) Kinetic studies J Biol Chem 240 2011–2018 Occurrence Handle1:CAS:528:DyaF2MXnsFKktw%3D%3D Occurrence Handle14299620
L Morpurgo E Agostinelli B Mondovi L Avigliano R Silvestri G Stefancich M Artico (1992) ArticleTitleBovine serum amine oxidase: Half-site reactivity with phenylhydrazine, semicarbazide, and aromatic hydrazides Biochemistry 31 2615–2621 10.1021/bi00124a023 Occurrence Handle10.1021/bi00124a023 Occurrence Handle1:CAS:528:DyaK38Xht1ektb0%3D Occurrence Handle1312354
M Mure SA Mills JP Klinman (2002) ArticleTitleCatalytic mechanism of the topa quinone containing copper amine oxidases Biochemistry 41 9269–9278 10.1021/bi020246b Occurrence Handle10.1021/bi020246b Occurrence Handle1:CAS:528:DC%2BD38XkslehsLc%3D Occurrence Handle12135347
A Padiglia R Medda A Lorrai M Paci JZ Pedersen A Boffi A Bellelli AF Agro G Floris (2001) ArticleTitleIrreversible inhibition of pig kidney copper-containing amine oxidase by sodium and lithium ions Eur J Biochem 268 4686–4697 10.1046/j.1432-1327.2001.02390.x Occurrence Handle10.1046/j.1432-1327.2001.02390.x Occurrence Handle1:CAS:528:DC%2BD3MXmslGntr4%3D Occurrence Handle11532005
J Plastino EL Green J Sanders-Loehr JP Klinman (1999) ArticleTitleAn unexpected role for the active site base in cofactor orientation and flexibility in the copper amine oxidase from Hansenula polymorpha Biochemistry 38 8204–8216 10.1021/bi9826660 Occurrence Handle10.1021/bi9826660 Occurrence Handle1:CAS:528:DyaK1MXjsVWmtrw%3D Occurrence Handle10387066
M Salmi S Jalkanen (2005) ArticleTitleCell-surface enzymes in control of leukocyte trafficking Nat Rev Immunol 5 760–771 10.1038/nri1705 Occurrence Handle10.1038/nri1705 Occurrence Handle1:CAS:528:DC%2BD2MXhtVGrurvF Occurrence Handle16200079
M Salmi G Yegutkin R Lehvonen K Koskinen T Salminen S Jalkanen (2001) ArticleTitleA cell surface amine oxidase directly controls lymphocyte migration Immunity 14 265–276 10.1016/S1074-7613(01)00108-X Occurrence Handle10.1016/S1074-7613(01)00108-X Occurrence Handle1:CAS:528:DC%2BD3MXis1ehurg%3D Occurrence Handle11290336
IH Segel (1993) Enzyme kinetics SeriesTitleBehavior and analysis of rapid equilibrium and steady-state enzyme systems Wiley Classics Library Edition Wiley New York
CM Stolen GG Yegutkin R Kurkijarvi P Bono K Alitalo S Jalkanen (2004) ArticleTitleOrigins of serum semicarbazide-sensitive amine oxidase Circ Res 95 50–57 10.1161/01.RES.0000134630.68877.2F Occurrence Handle10.1161/01.RES.0000134630.68877.2F Occurrence Handle1:CAS:528:DC%2BD2cXlt1Cru7k%3D Occurrence Handle15178639
F Vianello ML Di Paolo L Zennaro R Stevanato A Rigo (1992) ArticleTitleIsolation of amine oxidase from bovine plasma by a two-step procedure Protein Expres Purif 3 362–367 10.1016/S1046-5928(05)80036-1 Occurrence Handle10.1016/S1046-5928(05)80036-1 Occurrence Handle1:CAS:528:DyaK38Xmt1yrtbk%3D
CM Wilmot J Hajdu MJ McPherson PF Knowles SE Phillips (1999) ArticleTitleVisualization of dioxygen bound to copper during enzyme catalysis Science 286 1724–1728 10.1126/science.286.5445.1724 Occurrence Handle10.1126/science.286.5445.1724 Occurrence Handle1:CAS:528:DyaK1MXnslKmtL8%3D Occurrence Handle10576737
KD Yadav PF Knowles (1981) ArticleTitleA catalytic mechanism for benzylamine oxidase from pig plasma. Stopped- flow kinetic studies Eur J Biochem 114 139–144 Occurrence Handle1:CAS:528:DyaL3MXpvVOguw%3D%3D Occurrence Handle6260490 Occurrence Handle10.1111/j.1432-1033.1981.tb06184.x
PH Yu CY Fang CM Yang (1992) ArticleTitleSemicarbazide-sensitive amine oxidase from the smooth muscles of dog aorta and trachea: Activation by the MAO-A inhibitor clorgyline J Pharm Pharmacol 44 981–985 Occurrence Handle1:CAS:528:DyaK3sXltFyhsQ%3D%3D Occurrence Handle1361563
M Zhou N Panchuk-Voloshina (1997) ArticleTitleA one-step fluorometric method for the continuous measurement of monoamine oxidase activity Anal Biochem 237 169–174 10.1006/abio.1997.2392 Occurrence Handle10.1006/abio.1997.2392
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holt, A., Degenhardt, O., Berry, P. et al. The effects of buffer cations on interactions between mammalian copper-containing amine oxidases and their substrates. J Neural Transm 114, 733–741 (2007). https://doi.org/10.1007/s00702-007-0680-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-007-0680-1