Abstract
Purpose
This study was aimed to directly measure cerebrospinal fluid (CSF) gas tensions and pH before and after STA-MCA anastomosis for occlusive carotid artery diseases to investigate its direct effects on the ischemic brain.
Methods
This study included 9 patients who underwent STA-MCA anastomosis on the basis of CBF studies. About 1 mL of CSF was collected before and after bypass procedures, and CSF pH, CSF PO2, and CSF PCO2 were measured with a blood gas analyzer. As the controls, the CSF was collected from 6 patients during surgery for unruptured cerebral aneurysm. CSF PO2 and CSF PCO2 were expressed as the ratio to PaO2 and PaCO2, respectively.
Results
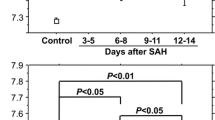
Before bypass procedure, CSF PO2/PaO2 was 0.88 ± 0.16, being lower than the controls (1.10 ± 0.09; P = 0.005). CSF PCO2/PaCO2 was 0.93 ± 0.13, being higher than the controls (0.84 ± 0.06; P = 0.039). Ipsilateral-to-contralateral CBF ratio had a positive correlation with CSF PO2/PaO2 (P = 0.0028) but a negative correlation with the CSF PCO2/PaCO2 (P = 0.0045). STA-MCA anastomosis increased CSF pH from 7.402 ± 0.133 to 7.504 ± 0.126 (P = 0.0011) and CSF PO2/PaO2 from 0.88 ± 0.16 to 1.05 ± 0.26 (P = 0.018) but decreased CSF PCO2/PaCO2 from 0.93 ± 0.13 to 0.70 ± 0.17 (P = 0.0006).
Conclusion
The severity of cerebral ischemia before surgery is intensely reflected in the gas tensions and pH of the CSF. STA-MCA anastomosis carries dramatic effects on CSF gas tensions and pH in hemodynamically compromised patients. CSF would be a valuable surrogate biomarker to monitor the severity of cerebral ischemia.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Anzai Y, Ishikawa M, Shaw DW, Artru A, Yarnykh V, Maravilla KR (2004) Paramagnetic effect of supplemental oxygen on CSF hyperintensity on fluid-attenuated inversion recovery MR images. AJNR Am J Neuroradiol 25:274–279
Brzezinski J, Kjallquist A, Siesjo BK (1967) Mean carbon dioxide tension in the brain after carbonic anhydrase inhibition. J Physiol 188:13–23
Christiansson L, Hellberg A, Svensson BA, Bergqvist D, Wiklund L, Karacagil S (2000) Relationship between intrathecal oxygen tension and ultrastructural changes in the spinal cord during experimental aortic clamping. Eur J Vasc Endovasc Surg 19:413–420
Czabanka M, Boschi A, Acker G, Pena-Tapia P, Schubert GA, Schmiedek P, Vajkoczy P (2016) Grading of moyamoya disease allows stratification for postoperative ischemia in bilateral revascularization surgery. Acta Neurochir (Wien) 158:1895–1900
Grubb RL Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, Spitznagel EL, Powers WJ (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280:1055–1060
Halmagyi DFJ, Gillett DJ (1967) Cerebrospinal fluid oxygen tension at different levels of oxygenation. Resp Physiol 2:207–212
Hollin SA, Espinosa OE, Sukoff MH, Jacobson JH 2nd (1968) The effect of hyperbaric oxygenation on cerebrospinal fluid oxygen. J Neurosurg 29:229–235
Jankowska L, Grieb P (1979) Relationship between arterial and cisternal CSF oxygen tension in rabbits. Am J Physiol 236:F220-225
Kashiwazaki D, Akioka N, Kuwayama N, Houkin K, Czabanka M, Vajkoczy P, Kuroda S (2017) Berlin grading system can stratify the onset and predict perioperative complications in adult moyamoya disease. Neurosurgery 81:986–991
Kazemi H, Klein RC, Turner FN, Strieder DJ (1968) Dynamics of oxygen transfer in the cerebrospinal fluid. Respir Physiol 4:24–31
Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H (2001) Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 32:2110–2116
Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K (1993) Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 32:912–918; discussion 918–919
Kuroda S, Kawabori M, Hirata K, Shiga T, Kashiwazaki D, Houkin K, Tamaki N (2014) Clinical significance of STA-MCA double anastomosis for hemodynamic compromise in post-JET/COSS era. Acta Neurochir (Wien) 156:77–83
Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N, Iwasaki Y (2006) Cerebral oxygen metabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery disease. Stroke 37:393–398
Kuroda S, Shiga T, Ishikawa T, Houkin K, Narita T, Katoh C, Tamaki N, Iwasaki Y (2004) Reduced blood flow and preserved vasoreactivity characterize oxygen hypometabolism due to incomplete infarction in occlusive carotid artery diseases. J Nucl Med 45:943–949
Lips J, de Haan P, Bouma GJ, Holman R, van Dongen E, Kalkman CJ (2005) Continuous monitoring of cerebrospinal fluid oxygen tension in relation to motor evoked potentials during spinal cord ischemia in pigs. Anesthesiology 102:340–345
Luh WM, Wong EC, Bandettini PA, Hyde JS (1999) QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 41:1246–1254
Lyu J, Duan Q, Xiao S, Meng Z, Wu X, Chen W, Wang G, Niu Q, Li X, Bian Y, Han D, Guo W, Yang S, Bian X, Lan Y, Wang L, Zhang T, Duan C, Zhang D, Wang X, Chen L, Tian C, Zhou X, Lou X, Investigators M-S (2023) Arterial spin labeling-based mri estimation of penumbral tissue in acute ischemic stroke. J Magn Reson Imaging 57:1241–1247
Maas AI, Fleckenstein W, de Jong DA, van Santbrink H (1993) Monitoring cerebral oxygenation: experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien) 59:50–57
Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ (2008) Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 39:2021–2028
Mori M, Chiba T, Nakamizo A, Kumashiro R, Murata M, Akahoshi T, Tomikawa M, Kikkawa Y, Yoshimoto K, Mizoguchi M, Sasaki T, Hashizume M (2014) Intraoperative visualization of cerebral oxygenation using hyperspectral image data: a two-dimensional mapping method. Int J Comput Assist Radiol Surg 9:1059–1072
Mrowka R (1981) Dynamics in changes in the acid-base equilibrium of arterial blood and the cerebrospinal fluid in condition of natural hyperventilation and apnoea. J Neurol Sci 49:181–191
Nemoto EM, Yonas H (2001) Revisiting the question, “is the acetazolamide test valid for quantitative assessment of maximal cerebral autoregulatory vasodilation?” Stroke 32:1234–1237
Ogasawara K, Ito H, Sasoh M, Okuguchi T, Kobayashi M, Yukawa H, Terasaki K, Ogawa A (2003) Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med 44:520–525
Ogasawara K, Ogawa A (2006) JET study (Japanese EC-IC bypass trial). Nippon Rinsho 64(Suppl 7):524–527
Powers WJ (1991) Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29:231–240
Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP (2011) Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 306:1983–1992
Powers WJ, Grubb RL Jr, Raichle ME (1984) Physiological responses to focal cerebral ischemia in humans. Ann Neurol 16:546–552
Rossanda M, Gordon E (1970) The oxygen tension of cerebrospinal fluid in patients with brain lesions. Acta Anaesthesiol Scand 14:173–181
Scheufler KM, Rohrborn HJ, Zentner J (2002) Does tissue oxygen-tension reliably reflect cerebral oxygen delivery and consumption? Anesth Analg 95:1042–1048, table of contents
Takahashi JC, Funaki T, Houkin K, Kuroda S, Fujimura M, Tomata Y, Miyamoto S (2020) Impact of cortical hemodynamic failure on both subsequent hemorrhagic stroke and effect of bypass surgery in hemorrhagic moyamoya disease: a supplementary analysis of the Japan Adult Moyamoya Trial. J Neurosurg 134:940–945
Ulus F, Hellberg A, Ulus AT, Karacagil S (2009) Alterations in cerebrospinal fluid PO(2), PCO(2), and pH measurements during and after experimental thoracic aortic cross-clamping. Ann Vasc Surg 23:122–127
Venkatesh B, Boots R, Tomlinson F, Jones RD (1999) The continuous measurement of cerebrospinal fluid gas tensions in critically ill neurosurgical patients: a prospective observational study. Intensive Care Med 25:599–605
Venkatesh B, Boots RJ (1997) Carbon dioxide and oxygen partial pressure measurements in the cerebrospinal fluid in a conventional blood gas analyzer: analysis of bias and precision. J Neurol Sci 147:5–8
Vorstrup S, Brun B, Lassen NA (1986) Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke 17:1291–1298
Vorstrup S, Henriksen L, Paulson OB (1984) Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Investig 74:1634–1639
Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, Yonekura Y, Konishi J, Kimura J (1996) Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry 61:18–25
Yonas H, Pindzola RR, Meltzer CC, Sasser H (1998) Qualitative versus quantitative assessment of cerebrovascular reserves. Neurosurgery 42:1005–1010; discussion 1011–1002
Acknowledgements
This study was partly supported by a grant from the Research Committee on Moyamoya Disease, sponsored by the Ministry of Health, Labor, and Welfare of Japan (Grant No. 23FC101).
Funding
This study was partly supported by a grant from the Research Committee on Moyamoya Disease, sponsored by the Ministry of Health, Labor, and Welfare of Japan (Grant No. 23FC101).
Author information
Authors and Affiliations
Contributions
SK contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by SK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was proved by the local institutional review board (IRB) and the written informed consent was obtained from all of participants in this study.
Consent for publication
This manuscript includes no data than can specify the individual information on the participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work has not been presented in any form and has not been published in any journals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuroda, S., Yamamoto, S., Hori, E. et al. Effects of superficial temporal artery to middle cerebral artery (STA-MCA) anastomosis on the cerebrospinal fluid gas tensions and pH in hemodynamically compromised patients. Acta Neurochir 165, 3709–3715 (2023). https://doi.org/10.1007/s00701-023-05855-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05855-5