Abstract
Background
The efficacy of the subthalamic nucleus (STN) stimulation for Parkinson’s disease has been widely established. The microlesion effect (MLE) due to deep brain stimulation (DBS) electrode implantation has been reputed to be a good predictor for long-term efficacy of the procedure but its analysis in asleep implantation is still unclear. We thus analyzed MLE rate in our strategy of targeting the STN on MRI under general anesthesia and its correlation with our long-term results.
Method
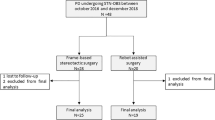
We retrospectively analyzed 32 consecutive parkinsonian patients implanted with a DBS targeting the STN bilaterally under general anesthesia between October 2013 and December 2020. Targeting was performed after head frame and localizer placement using a stereotactic peroperative robotic 3D fluoroscopy (Artis Zeego, Siemens) fused with preoperative CT and MRI data. We collected intraoperative data, postoperative occurrence of MLE, modification of Unified Parkinson Disease Rating Scale item III (UPDRS III) postoperatively and at subsequent visit, as well as reduction of medication.
Results
The mean operative time was 223 min. No permanent complication occurred. MLE was observed in 90.7%. The mean follow-up time was 17 months. The UPDRS III for the off medication/on stimulation condition improved by 64.8% from baseline. The mean dose reduction of Prolopa after the surgical procedure was 31.3%.
Conclusions
Direct targeting of STN under general anesthesia based on preoperative CT and MRI data fused with a preoperative 3D fluoroscopy is safe. It allows for a high rate of postoperative MLE (90.7%) and results in prolonged clinical improvement.





Similar content being viewed by others
References
Cersosimo MG, Raina GB, Benarroch EE, Piedimonte F, Alemán GG, Micheli FE (2009) Micro lesion effect of the globus pallidus internus and outcome with deep brain stimulation in patients with Parkinson disease and dystonia. Mov Disord 24(10):1488–1493
Chang SY, Shon YM, Agnesi f, Lee KH (2009) Microthalamotomy effect during deep brain stimulation: potential involvement of adenosine and glutamate efflux. Proc 31 st Annu Int Conf IEEE Eng Med Biol Soc Eng Futur Biomed EMBC:3294–3297
Charles PD, Van Blercom N, Krack P, Lee SL, Xie J, Besson G, Benabid AL, Pollak P (2002) Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology 59:932–934
Delavallée M, Delaunois J, Ruwet J, Jeanjean A, Raftopoulos C (2016) STN DBS for Parkinson’s disease: results from a series of ten consecutive patients implanted under general anaesthesia with intraoperative use of 3D fluoroscopy to control lead placement. Acta Neurochir (Wien) 158(9):1783–1788
Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE, Rodriguez-Oroz MC, Tamma F, Tröster AI, Vitek JL, Volkmann J, Voon V (2006) Deep brain stimulation: postoperative issues. Mov Disord 21(Suppl 14):S219–S237
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group, Neurostimulation Section (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266
Goodman RR, Kim B, McClelland S 3rd et al (2006) Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 77:12–17
Granziera C, Pollo C, Russmann H, Staedler C, Ghika J, Villemure JG, Burkhard PR, Vingerhoets FJ (2008) Sub-acute delayed failure of subthalamic DBS in Parkinson’s disease: the role of micro-lesion effect. Parkinsonism Relat Disord 14(2):109–113
Ho AL, Ali R, Connolly ID, Henderson JM, Dhall R, Stein SC, Halpern CH (2018) Awake versus asleep deep brain stimulation for Parkinson’s disease: a critical comparison and meta-analysis. J Neurol Neurosurg Psychiatry (7):687–691
Holiga S, Mueller K, Möller HE et al (2015) Resting-state functional magnetic resonance imaging of the subthalamic microlesion and stimulation effects in Parkinson’s disease: indications of a principal role of the brainstem. Neuroimage Clin 9:264–274
Holloway K, Docef A (2013) A quantitative assessment of the accuracy and reliability of O-arm images for deep brain stimulation surgery. Neurosurgery 72(1 Suppl Operative):47–57
Jech R, Mueller K, Urgošík D, Sieger T, Holiga Š, Růžička F, Dušek P, Havránková P, Vymazal J, Růžička E (2012) The subthalamic microlesion story in Parkinson’s disease: electrode insertion-related motor improvement with relative cortico-subcortical hypoactivation in fMRI. PLoS One 7(11)
Lange SF, Kremer NI, van Laar T, Lange F, Steendam-Oldekamp TE, Oterdoom DLM, Absalom AR, van Dijk JMC, Drost G (2021) The intraoperative microlesion effect positively correlates with the short-term clinical effect of deep brain stimulation in Parkinson’s disease. Neuromodulation. Online ahead of print. https://doi.org/10.1111/ner.13523
Lyons KE, Wilkinson SB, Overman J, Pahwa R (2004) Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology 63:612–616
Maltête D, Navarro S, Welter ML, Roche S, Bonnet AM, Houeto JL, Mesnage V, Pidoux B, Dormont D, Cornu P, Agid Y (2004) Subthalamic stimulation in Parkinson disease: with or without anesthesia? Arch Neurol 61(3):390–392
Maltête D, Derrey S, Chastan N, Debono B, Gérardin E, Fréger P, Mihout B, Menard JF, Hannequin D (2008) Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Mov Disord 23(7):1047–1050
Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, Pahwa R, Lyons KE, Tröster AI, Vitek JL, Tagliati M, SJM DBS Study Group (2012) Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 11(2):140–149
Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, Pahwa R, Lyons KE, Tröster AI, Vitek JL, Tagliati M, SJM DBS Study Group (2012) Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 11(2):140–9
Pilitsis JG, Rezai AR, Boulis NM, Henderson JM, Busch RM, Kubu CS (2005) A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson’s disease. Stereotact Funct Neurosurg 83:67–70
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, BrefelCourbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltête D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Krüger R, Pinsker MO, Amtage F, Régis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, EARLYSTIM Study Group (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–22
Tolleson C, Stroh J, Ehrenfeld J, Neimat J, Konrad P, Phibbs F (2014) The factors involved in deep brain stimulation infection: a large case series. Stereotact Funct Neurosurg 92(4):227–233
Tsai ST, Lin SH, Chou YC, Pan YH, Hung HY, Li CW, Lin SZ, Chen SY (2009) Prognostic factors of subthalamic stimulation in Parkinson’s disease: a comparative study between short- and long-term effects. Stereotact Funct Neurosurg 87(4):241–248
Tykocki T, Nauman P, Koziara H, Mandat T (2013) Microlesion effect as a predictor of the effectiveness of subthalamic deep brain stimulation for Parkinson’s disease. Stereotact Funct Neurosurg 91(1):12–17. https://doi.org/10.1159/000342161
Vitek JL, Jain R, Chen L, Tröster AI, Schrock LE, House PA, Giroux ML, Hebb AO, Farris SM, Whiting DM, Leichliter TA, Ostrem JL, San Luciano M, Galifianakis N, VerhagenMetman L, Sani S, Karl JA, Siddiqui MS, Tatter SB, UlHaq I, Machado AG, Gostkowski M, Tagliati M, Mamelak AN, Okun MS, Foote KD, Moguel-Cobos G, Ponce FA, Pahwa R, Nazzaro JM, Buetefisch CM, Gross RE, Luca CC, Jagid JR, Revuelta GJ, Takacs I, Pourfar MH, Mogilner AY, Duker AP, Mandybur GT, Rosenow JM, Cooper SE, Park MC, Khandhar SM, Sedrak M, Phibbs FT, Pilitsis JG, Uitti RJ, Starr PA (2020) Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol 19(6):491–550
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, PD SURG Collaborative Group (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9(6):581–91
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Local Ethics Committee (Comité d’Ethique Hospitalo-Facultaire de l’Université catholique de Louvain, CE B 403) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all patients included into the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery—Movement disorders
Rights and permissions
About this article
Cite this article
Soler-Rico, M., Peeters, JB., Joris, V. et al. MRI-guided DBS of STN under general anesthesia for Parkinson’s disease: results and microlesion effect analysis. Acta Neurochir 164, 2279–2286 (2022). https://doi.org/10.1007/s00701-022-05302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05302-x