AbstractAbstract
Background
Perineural spread (PNS) of tumors from pelvic malignancies is a rare phenomenon but constitutes an important differential diagnosis of lumbosacral plexopathy (LSP). Herein, we describe the clinical and imaging features of patients with LSP due to PNS of pelvic malignancies along with a literature review.
Methods
We retrospectively reviewed 9 cases of LSP caused by PNS of pelvic malignancy between January 2006 and August 2021, and all clinical and imaging parameters were recorded in detail. Clinical symptoms and signs of patients were described and listed in the order in which they occurred. The results of imaging test were analyzed to describe specific findings in LSP caused by PNS.
Results
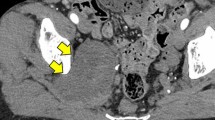
This study enrolled nine adult patients (mean age, 50.1 years). Two cases initially presented as LSP and were later diagnosed with pelvic malignancy. Pain in the perianal or inguinal area preceded pain at the extremities in six patients. Neurogenic bladder or bowel symptoms developed in five patients. On the magnetic resonance imaging (MRI), the S1-S2 spinal nerve was most commonly involved, and S1 myotome weakness was more prominent in six patients than the other myotomes. One patient had an intradural extension. 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) and computed tomography (CT) showed abnormal signal intensity in six patients. No abnormality in 18F-FDG PET/CT was detected in the nervous structures in one patient. Only four patients survived until the last follow-up visit.
Conclusions
Though rare, physicians should always keep in mind the possibility of LSP due to the PNS in patients with pelvic malignancy. Thorough physical examination and history taking could provide clues for diagnosis. Pelvic MRI and 18F-FDG-PET/CT should be considered for patients with LSP to rule out neoplastic LSP.





Similar content being viewed by others
Change history
23 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00701-022-05285-9
Abbreviations
- PNS:
-
Perineural spread
- LSP:
-
Lumbosacral plexopathy
- RIP:
-
Radiation-induced plexopathy
- MRI:
-
Magnetic resonance image
- PNI:
-
Perineural invasion
- FDG:
-
Fluorodeoxyglucose
- PET/CT:
-
Positron emission tomography/computed tomography
References
Aghion DM, Capek S, Howe BM, Hepel JT, Sambandam S, Oyelese AA, Spinner RJ (2014) Perineural tumor spread of bladder cancer causing lumbosacral plexopathy: an anatomic explanation. Acta Neurochir (Wien) 156:2331–2336
Antoine JC, Camdessanché JP (2007) Peripheral nervous system involvement in patients with cancer. Lancet Neurol 6:75–86
Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49:213–223
Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D (2004) Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res 64:6082–6090
Babu MA, Spinner RJ, Dyck PJ, Amrami KK, Nathan MA, Kawashima A, Howe BM (2013) Recurrent prostatic adenocarcinoma with perineural spread to the lumbosacral plexus and sciatic nerve: comparing high resolution MRI with torso and endorectal coils and F-18 FDG and C-11 choline PET/CT. Abdom Imaging 38:1155–1160
Beard CJ, Chen MH, Cote K, Loffredo M, Renshaw AA, Hurwitz M, D’Amico AV (2004) Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys 58:19–24
Bellis D, Marci V, Monga G (1993) Light microscopic and immunohistochemical evaluation of vascular and neural invasion in colorectal cancer. Pathol Res Pract 189:443–447
Caldemeyer KS, Mathews VP, Righi PD, Smith RR (1998) Imaging features and clinical significance of perineural spread or extension of head and neck tumors. Radiographics 18:97–110
Capek S, Howe BM, Amrami KK, Spinner RJ (2015) Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg Focus 39:E14
Capek S, Howe BM, Tracy JA, García JJ, Amrami KK, Spinner RJ (2015) Prostate cancer with perineural spread and dural extension causing bilateral lumbosacral plexopathy: case report. J Neurosurg 122:778–783
Capek S, Sullivan PS, Howe BM, Smyrk TC, Amrami KK, Spinner RJ, Dozois EJ (2015) Recurrent rectal cancer causing lumbosacral plexopathy with perineural spread to the spinal nerves and the sciatic nerve: an anatomic explanation. Clin Anat 28:136–143
Elsahwi KS, Barber E, Illuzzi J, Buza N, Ratner E, Silasi DA, Santin AD, Azodi M, Schwartz PE, Rutherford TJ (2011) The significance of perineural invasion in early-stage cervical cancer. Gynecol Oncol 123:561–564
Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT (1998) Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 124:637–640
Gwathmey KG (2018) Plexus and peripheral nerve metastasis. Handb Clin Neurol 149:257–279
Harper CM Jr, Thomas JE, Cascino TL, Litchy WJ (1989) Distinction between neoplastic and radiation-induced brachial plexopathy, with emphasis on the role of EMG. Neurology 39:502–506
Hebert-Blouin MN, Amrami KK, Myers RP, Hanna AS, Spinner RJ (2010) Adenocarcinoma of the prostate involving the lumbosacral plexus: MRI evidence to support direct perineural spread. Acta Neurochir (Wien) 152:1567–1576
Howe BM, Amrami KK, Nathan MA, Garcia JJ, Spinner RJ (2013) Perineural spread of cervical cancer to the sciatic nerve. Skeletal Radiol 42:1627–1631
Horn A, Dahl O, Morild I (1991) Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum 34:798–804
Jaeckle KA (1991) Nerve plexus metastases. Neurol Clin 9:857–866
Jaeckle KA (2004) Neurological manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol 24:385–393
Jaeckle KA, Young DF, Foley KM (1985) The natural history of lumbosacral plexopathy in cancer. Neurology 35:8–15
Kimura K, Tsuchiya J, Manabe O, Yokoyama K, Toshiaki I, Tateishi U (2021) Perineural spread of gastric cancer to the sciatic nerve incidentally detected by (18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging 48:940–941
Ladha SS, Spinner RJ, Suarez GA, Amrami KK, Dyck PJ (2006) Neoplastic lumbosacral radiculoplexopathy in prostate cancer by direct perineural spread: an unusual entity. Muscle Nerve 34:659–665
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115:3379–3391
Maru N, Ohori M, Kattan MW, Scardino PT, Wheeler TM (2001) Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol 32:828–833
Memarzadeh S, Natarajan S, Dandade DP, Ostrzega N, Saber PA, Busuttil A, Lentz SE, Berek JS (2003) Lymphovascular and perineural invasion in the parametria: a prognostic factor for early-stage cervical cancer. Obstet Gynecol 102:612–619
Murthy NK, Amrami KK, Spinner RJ (2020) Perineural spread to the brachial plexus: a focused review of proposed mechanisms and described pathologies. Acta Neurochir (Wien) 162:3179–3187
Oñate-Ocaña LF, Montesdeoca R, López-Graniel CM, Aiello-Crocifoglio V, Mondragón-Sánchez R, Cortina-Borja M, Herrera-Goepfert R, Oros-Ovalle C, Gallardo-Rincón D (2004) Identification of patients with high-risk lymph node-negative colorectal cancer and potential benefit from adjuvant chemotherapy. Jpn J Clin Oncol 34:323–328
Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y, Yamauchi H, Ogata Y (1999) The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today 29:16–22
Siquara De Sousa AC, Capek S, Amrami KK, Spinner RJ (2015) Neural involvement in endometriosis: Review of anatomic distribution and mechanisms. Clin Anat 28:1029–1038
Stone JJ, Adamo DA, Khan DZ, Packard AT, Broski SM, Nathan MA, Howe BM, Spinner RJ (2019) Multimodal imaging aids in the diagnosis of perineural spread of prostate cancer. World Neurosurg 122:e235–e240
Schaller B, Merlo A, Kirsch E, Lehmann K, Huber PR, Lyrer P, Steck AJ, Gratzl O (1998) Prostate-specific antigen in the cerebrospinal fluid leads to diagnosis of solitary cauda equina metastasis: a unique case report and review of the literature. Br J Cancer 77:2386–2389
Thomas JE, Cascino TL, Earle JD (1985) Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology 35:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to participate
Informed consent was waived because of the retrospective nature of the study and because the analysis used anonymous clinical data.
Conflict of interest
The authors declare no competing interests.
Additional information
Comments:
The paper by Lee et al. describes 9 patients collected over 15 years with lumbosacral plexopathy (LSP) associated with a variety of pelvic malignancies (e.g., prostate, cervical and rectal) and perineural spread. It is gratifying to see some of our contributions (1) not only corroborated but extended. This case series supports the notion that the entity of neoplastic lumbosacral plexopathy is not as rare as thought: being underdiagnosed, misdiagnosed and underreported. In the past, these patients were assumed to have LSP due to radiation, chemotherapy, or inflammation or that is idiopathic.
The neural highways (2) underlying neoplastic LSP originate at the affected pelvic organ (or staple line) where perineural transitions to perineural spread. Perineural spread continues via pelvic autonomic nerves (i.e., inferior hypogastric plexus) to the lumbosacral plexus and can extend proximally to spinal nerves, dura, and, even to the other limb (3) and/or distally to the sciatic nerve. Perineural spread can go considerable distances (4). Clinical presentations and radiologic patterns are emerging based on the pathoanatomy and sequential features can be interpreted based on the pathoanatomic findings.
In this paper, neoplastic LSP was established by radiologic features (enlarged nerves with increased T2 and perifascicular enhancement on MRI; often with increased avidity on PET). Only 2 patients had tissue confirmation. At our institution, we believe “Tissue is the issue” and utilize the technique of image-guided targeted fascicular biopsy (5) whenever feasible. We search for an extrapelvic site whenever possible and often identify blue infiltrates within the nerve (6). Occasionally, we are forced to treat patients empirically who are thought to have perineural spread of a pelvic cancer but confirmatory tissue is unobtainable and suspicion is high.
Several words of caution. Radiologic findings by themselves can be non-specific. One cannot establish diagnosis on T2-weighted images alone and patterns of contrast enhancement may be variable with few truly pathognomonic features that would negate the need for tissue diagnosis. We suggest PET-CT or MR correlation in all cases (note in this series, only 7 of 9 patients had PET and 1 of these PET studies was not avid). The radiologic differential diagnosis is broad. We have treated several patients with imaging features who were thought to have perineural spread from the primary cancer but who were found to have a radiation-induced malignant peripheral nerve sheath tumor (7) (note 5 patients in this series had previous radiation). Furthermore, the presence of myokymic discharges on EMG (which is supportive of a diagnosis of radiation plexopathy and was used to exclude potential patients in this series) does not exclude concomitant cancer.
Ultimately optimal targeted treatment must consider the specific diagnosis, the mechanism (perineural spread) and the extent of disease (with/or without concomitant distant hematogenous or lymphogenous spread). Surgical resection is often not feasible. Adjuvant therapy needs to be considered.
Perineural spread is well known when involving the head/neck with certain types of cancer in particular (e.g., melanoma, squamous cell carcinoma, etc). It is time to expand our knowledge to other sites, including the lumbosacral plexus and brachial plexus, and other types of cancers (8,9).
Robert J. Spinner
Kimberly K. Amrami
Rochester, MN, USA
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor-Other
Rights and permissions
About this article
Cite this article
Lee, B.C., Kim, S.W., Kim, D.H. et al. Lumbosacral plexopathy caused by the perineural spread of pelvic malignancies: clinical aspects and imaging patterns. Acta Neurochir 164, 1509–1519 (2022). https://doi.org/10.1007/s00701-022-05194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05194-x