Abstract
Background
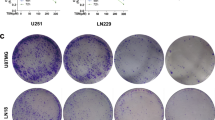
Our current understanding of the role of dequalinium chloride (DECA) in the progression of glioma remains very limited. This study was aimed to investigate the effect of DECA on human glioma cell lines in vitro and vivo.
Methods
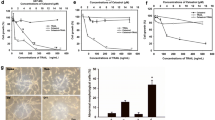
The underlying molecular mechanism was analyzed for developing potential targeted agents. MTT assay, genomic DNA electrophoresis, DAPI staining, TUNEL staining, and wound scratch assay were performed to evaluate the effect of DECA on human glioma cell lines. Bioinformatics methods were used to screen the possible signaling pathway proteins, and the expression of these proteins and the corresponding mRNA was measured.
Results
DECA significantly inhibited the growth and proliferation of human glioma cells. Screening of apoptosis-related proteins showed the mRNA expression level of 6 genes was significantly changed after DECA administration.
Conclusion
This study shows that DECA effectively inhibits the growth of glioma cells in vitro and vivo. DECA may promote glioma cell apoptosis by affecting the expression of NFKB2, HRAS, NF1, CBL, RAF1, and BCL-2 genes.




Similar content being viewed by others
Abbreviations
- DECA:
-
Dequalinium chloride
- TMZ:
-
Temozolomide
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- T/C:
-
The relative tumor proliferation rate
- RTV:
-
Relative tumor volume
- IC50:
-
The median inhibitory concentration
References
Anton K, Baehring JM, Mayer T (2012) Glioblastoma multiforme overview of current treatment and future perspectives. Hematol Oncol Clin North Am 26:825–853
Bondy ML, Scheurer ME, Beatrice M, Barnholtz-Sloan JS, Davis FG, Dora IY, Carol K, Mccarthy BJ, Preetha R, Schwartzbaum JA (2010) Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 113:1953–1968
Chen F, Chen B, Wang H, Xu W, Li W, Chen D (2018) Intracranial nonskull-based chondrosarcoma arising from the sagittal sinus: a case report and review of the literature. World Neurosurg 120:234–239. https://doi.org/10.1016/j.wneu.2018.08.239
Cheng-Zheng W, Peng Y, Yin Y (2015) MiR-126 regulated breast cancer cell invasion by targeting ADAM9. Int J Clin Exp Pathol 8:6547–6553
Daniel MM, Meritxell N, María Carmen LC, Amaia E, Marta P, Niccolò T, Marina DB, María R, Emili M, Alvaro UIJO (2014) XIAP inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells. Oncotarget 5:4337
D'Auria FD, Simonetti G, Strippoli V (1989) Antimicrobial characteristics of a tincture of dequalinium chloride. Ann Ig 1:1227
Della Casa V, Noll H, Gonser S, Grob P, Graf F, Pohlig G (2002) Antimicrobial activity of dequalinium chloride against leading germs of vaginal infections. Arzneimittelforschung 52:699–705
Elmore LW, Holt SE (2000) Telomerase and telomere stability: a new class of tumor suppressor? Mol Carcinog 28:1–4
Faje AT, Nachtigall L, Wexler D, Miller KK, Klibanski A, Makimura H (2013) Central diabetes insipidus: a previously unreported side effect of temozolomide. J Clin Endocrinol Metab 98:3926–3931
Galeano E, Nieto E, García-Pérez AI, Delgado MD, Pinilla M, Sancho P (2005) Effects of the antitumoural dequalinium on NB4 and K562 human leukemia cell lines : mitochondrial implication in cell death. Leuk Res 29:1201–1211
García-Pérez AI, Galeano E, Nieto E, Sancho P (2011) Dequalinium induces human leukemia cell death by affecting the redox balance. Leuk Res 35:1395–1401
Green SL, Giaccia AJ (1998) Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J Sci Am 4:218–223
Mannas JP, Lightner DD, Defrates SR, Pittman T, Villano JL (2014) Long-term treatment with temozolomide in malignant glioma. J Clin Neurosci 21:121–123. https://doi.org/10.1016/j.jocn.2013.03.039
Marx S, Xiao Y, Baschin M, Splittstohser M, Altmann R, Moritz E, Jedlitschky G, Bien-Moller S, Schroeder HWS, Rauch BH (2019) The role of platelets in cancer pathophysiology: focus on malignant Glioma. Cancers 11. https://doi.org/10.3390/cancers11040569
Mendling W, Weissenbacher ER, Gerber S, Prasauskas V, Grob P (2016) Use of locally delivered dequalinium chloride in the treatment of vaginal infections: a review. Arch Gynecol Obstet 293:469–484
Ortega R, Bresson C, Darolles C, Gautier C, Roudeau S, Perrin L, Janin M, Floriani M, Aloin V, Carmona A, Malard V (2014) Low-solubility particles and a Trojan-horse type mechanism of toxicity: the case of cobalt oxide on human lung cells. Part Fibre Toxicol 11:14–14
Ostrom QT, Haley G, Paul F, Annie O, Yanwen C, Yingli W, Stroup NE, Carol K, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15. https://doi.org/10.1093/neuonc/not151
Park R, Chang CC, Liang YC, Chung Y, Henry RA, Lin E, Mold DE, Huang RC (2005) Systemic treatment with tetra-O-methyl nordihydroguaiaretic acid suppresses the growth of human xenograft tumors. Clin Cancer Res 11:4601–4609. https://doi.org/10.1158/1078-0432.Ccr-04-2188
Sancho P, Galeano E, Nieto E, Delgado MD, García-Pérez AI (2007) Dequalinium induces cell death in human leukemia cells by early mitochondrial alterations which enhance ROS production. Leuk Res 31:969–978
Sauvage F, Legrand FX, Roux M, Rajkovic I, Weiss TM, Varga Z, Augis L, Nugue G, Debouzy JC, Vergnaud-Gauduchon J, Barratt G (2019) Interaction of dequalinium chloride with phosphatidylcholine bilayers: a biophysical study with consequences on the development of lipid-based mitochondrial nanomedicines. J Colloid Interface Sci 537:704–715. https://doi.org/10.1016/j.jcis.2018.11.059
Slattum GM, Rosenblatt J (2014) Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer 14:495–501
Sullivan RM, Stone M, Marshall JF, Uberall F, Rotenberg SA (2000) Photo-induced inactivation of protein kinase calpha by dequalinium inhibits motility of murine melanoma cells. Mol Pharmacol 58:729
Weiss MJ, Wong JR, Ha CS, Bleday R, Salem RR, Steele GD, Chen LB (1987) Dequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulation. Proc Natl Acad Sci U S A 84:5444–5448
Wongrakpanich A, Geary SM, Joiner MA, Anderson ME, Salem AK (2014) Mitochondria-targeting particles. Nanomedicine (Lond) 9:2531–2543
Funding
Open Access funding provided by Projekt DEAL. Jilin Province Health and Family Planning Commission’s Science and Technology Enhancement Plan provided financial support in the form of cash funding (NO. 2017J045). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
“All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Yu, Y., Yang, B., Yu, J. et al. Dequalinium chloride inhibits the growth of human glioma cells in vitro and vivo: a study on molecular mechanism and potential targeted agents. Acta Neurochir 162, 1683–1690 (2020). https://doi.org/10.1007/s00701-020-04401-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04401-x