Abstract
Background
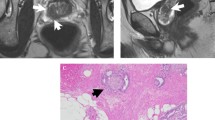
Perineural spread (PNS) of pelvic cancer along the lumbosacral plexus is an emerging explanation for neoplastic lumbosacral plexopathy (nLSP) and an underestimated source of patient morbidity and mortality. Despite the increased incidence of PNS, these patients are often times a clinical conundrum—to diagnose and to treat. Building on previous results in modeling glioblastoma multiforme (GBM), we present a mathematical model for predicting the course and extent of the PNS of recurrent tumors.
Methods
We created three-dimensional models of perineurally spreading tumor along the lumbosacral plexus from consecutive magnetic resonance imaging scans of two patients (one each with prostate cancer and cervical cancer). We adapted and applied a previously reported mathematical model of GBM to progression of tumor growth along the nerves on an anatomical model obtained from a healthy subject.
Results
We were able to successfully model and visualize perineurally spreading pelvic cancer in two patients; average growth rates were 60.7 mm/year for subject 1 and 129 mm/year for subject 2. The model correlated well with extent of PNS on MRI scans at given time points.
Conclusions
This is the first attempt to model perineural tumor spread and we believe that it provides a glimpse into the future of disease progression monitoring. Every tumor and every patient are different, and the possibility to report treatment response using a unified scale—as “days gained”—will be a necessity in the era of individualized medicine. We hope our work will serve as a springboard for future connections between mathematics and medicine.




Similar content being viewed by others
References
Aghion DM, Capek S, Howe BM, Hepel JT, Sambandam S, Oyelese AA, Spinner RJ (2014) Perineural tumor spread of bladder cancer causing lumbosacral plexopathy: an anatomic explanation. Acta Neurochir 156:2331–2336
Ayati B, Graham J, Holstein S (2012) Using a level set to model multiple myeloma induced bone loss. Proceedings of the 6th European Congress on Computational Methods in Applied Sciences and Engineering, University of Vienna, 10-14 September 2012
Baldock AL, Rockne RC, Boone AD, Neal ML, Hawkins-Daarud A, Corwin DM, Bridge CA, Guyman LA, Trister AD, Mrugala MM, Rockhill JK, Swanson KR (2013) From patient-specific mathematical neuro-oncology to precision medicine. Front Oncol 3:62
Belfatto A, Riboldi M, Ciardo D, Cattani F, Cecconi A, Lazzari R, Jereczek-Fossa BA, Orecchia R, Baroni G, Cerveri P (2016) Kinetic models for predicting cervical cancer response to radiation therapy on individual basis using tumor regression measured in vivo with volumetric imaging. Technol Cancer Res Treat 15:146–158
Capek S, Howe BM, Amrami KK, Spinner RJ (2015) Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg Focus 39:E14
Capek S, Howe BM, Tracy JA, Garcia JJ, Amrami KK, Spinner RJ (2015) Prostate cancer with perineural spread and dural extension causing bilateral lumbosacral plexopathy: case report. J Neurosurg 122:778–783
Capek S, Sullivan PS, Howe BM, Smyrk TC, Amrami KK, Spinner RJ, Dozois EJ (2015) Recurrent rectal cancer causing lumbosacral plexopathy with perineural spread to the spinal nerves and the sciatic nerve: an anatomic explanation. Clin Anat 28:136–143
Cocosco CA, Kollokian V, Kwan RK-S, Pike GB, Evans AC (1997) Brainweb: Online interface to a 3D MRI simulated brain database. http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=B637F41C2117EAC747DCE30E77F42A24?doi=10.1.1.51.3917&rep=rep1&type=pdf. Accessed 8 Oct 2017
Fisher RA (1937) The wave of advance of advantageous genes. Ann Hum Genet 7:355–369
Gerin C, Pallud J, Deroulers C, Varlet P, Oppenheim C, Roux FX, Chretien F, Thomas SR, Grammaticos B, Badoual M (2013) Quantitative characterization of the imaging limits of diffuse low-grade oligodendrogliomas. Neuro-Oncology 15:1379–1388
Gordillo N, Montseny E, Sobrevilla P (2013) State of the art survey on MRI brain tumor segmentation. Magn Reson Imaging 31:1426–1438
Howe BM, Amrami KK, Nathan MA, Garcia JJ, Spinner RJ (2013) Perineural spread of cervical cancer to the sciatic nerve. Skelet Radiol 42:1627–1631
Klein A, Tourville J (2012) 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 6:171
Konukoglu E (2009) Modeling glioma growth and personalizing growth models in medical images. Thesis, University of Nice
Konukoglu E, Clatz O, Delingette H, Ayache N (2010) Personalization of reaction-diffusion tumor growth models in MR images: application to brain gliomas characterization and radiotherapy planning. In: Deisboeck T, Stamatakos GS (eds) Multiscale cancer modeling. CRC Press, Boca Raton, pp 385–405
Konukoglu E, Clatz O, Menze BH, Stieltjes B, Weber MA, Mandonnet E, Delingette H, Ayache N (2010) Image guided personalization of reaction-diffusion type tumor growth models using modified anisotropic eikonal equations. IEEE Trans Med Imaging 29:77–95
Ladha SS, Spinner RJ, Suarez GA, Amrami KK, Dyck PJ (2006) Neoplastic lumbosacral radiculoplexopathy in prostate cancer by direct perineural spread: an unusual entity. Muscle Nerve 34:659–665
Malek A (2013) Experimental metastasis: modeling and analysis. Springer, Dordrecht
Neal ML, Trister AD, Ahn S, Baldock A, Bridge CA, Guyman L, Lange J, Sodt R, Cloke T, Lai A, Cloughesy TF, Mrugala MM, Rockhill JK, Rockne RC, Swanson KR (2013) Response classification based on a minimal model of glioblastoma growth is prognostic for clinical outcomes and distinguishes progression from pseudoprogression. Cancer Res 73:2976–2986
Neal ML, Trister AD, Cloke T, Sodt R, Ahn S, Baldock AL, Bridge CA, Lai A, Cloughesy TF, Mrugala MM, Rockhill JK, Rockne RC, Swanson KR (2013) Discriminating survival outcomes in patients with glioblastoma using a simulation-based, patient-specific response metric. PLoS One 8:e51951
Neal RE 2nd, Millar JL, Kavnoudias H, Royce P, Rosenfeldt F, Pham A, Smith R, Davalos RV, Thomson KR (2014) In vivo characterization and numerical simulation of prostate properties for non-thermal irreversible electroporation ablation. Prostate 74:458–468
Rockne R, Alvord EC Jr, Reed P, Swanson KR (2008) Modeling the growth and invasion of gliomas, from simple to complex: the goldie locks paradigm. Biophys Rev Lett 3:111–123
Scott JG, Basanta D, Anderson AR, Gerlee P (2013) A mathematical model of tumour self-seeding reveals secondary metastatic deposits as drivers of primary tumour growth. J R Soc Interface 10:20130011
Scott JG, Gerlee P, Basanta D, Fletcher AG, Maini PK, Anderson AR (2013) Mathematical modeling of the metastatic process. In: Malek A (ed) Experimental metastasis: modeling and analysis. Springer, Dordrecht, pp 189–208
Sethian JA (1999) Level set methods and fast marching methods. Cambridge University Press, Cambridge
Sun X, Bao J, Nelson KC, Li KC, Kulik G, Zhou X (2013) Systems modeling of anti-apoptotic pathways in prostate cancer: psychological stress triggers a synergism pattern switch in drug combination therapy. PLoS Comput Biol 9:e1003358
Swanson KR (1999) Mathematical modeling of the growth and control of tumors. University of Washington, Seattle
Swanson KR, Bridge C, Murray JD, Alvord EC Jr (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216:1–10
Swanson KR, Harpold HL, Peacock DL, Rockne R, Pennington C, Kilbride L, Grant R, Wardlaw JM, Alvord EC Jr (2008) Velocity of radial expansion of contrast-enhancing gliomas and the effectiveness of radiotherapy in individual patients: a proof of principle. Clin Oncol (R Coll Radiol) 20:301–308
Unkelbach J, Menze BH, Konukoglu E, Dittmann F, Le M, Ayache N, Shih HA (2014) Radiotherapy planning for glioblastoma based on a tumor growth model: improving target volume delineation. Phys Med Biol 59:747–770
Xie K, Yang J, Zhang ZG, Zhu YM (2005) Semi-automated brain tumor and edema segmentation using MRI. Eur J Radiol 56:12–19
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31:1116–1128
Funding
National Institute of Health provided financial support in the form NIH grant R01 CA 164371. The sponsor had no role in the design or conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Additional information
Comments
An interesting article about applying a mathematical model to quantify the perineural spread of tumor in two clinical cases over several time points. It is a first step with many pitfalls, such as the variable growth rate of tumors over time, that are both mentioned and discussed. Nevertheless, it is an important early first step towards more precisely describing tumor growth that hopefully will eventually make it possible to better assess treatment impact as well as clinical prognosis such as survival.
Michel Kliot
CA, USA
Electronic supplementary material
Video 1
Subject 1: simulated progression of perineural spread of prostate cancer. This video shows 3D visualization of perineurally spreading prostate cancer in subject 1. Growth is projected on an atlas images derived from a healthy subject; the simulation starts at a presumed point of entry of the cancer cells to the lumbosacral plexus (day 0) and continues “into the future” to emulate progression of an untreated tumor. (AVI 15172 kb)
Video 2
Subject 2: simulated progression of perineural spread of cervical cancer. This video shows 3D visualization of perineurally spreading cervical cancer in subject 2. Growth is projected on an atlas images derived from a healthy subject; the simulation starts at a presumed point of entry of the cancer cells to the lumbosacral plexus (day 0) and continues “into the future” to emulate progression of an untreated tumor. (AVI 6463 kb)
Appendix 1
Appendix 1
We adapted a mathematical model of GBM [27] to simulate PNS. The model is based on the Fisher-Kolmogorov equation (Eq. 1) [9],
where the solution variable c(x, t) represents the cancer cell density for spatial location x and time t. The parameters are the spatially varying invasion coefficient D, the proliferation rate ρ, and the carrying capacity of the tissue K. The spatial boundaries are given by the outer limits of the nerves. This is expressed in Eq. 2.
with the epineurium representing a no-flux boundary for the growth of the perineural cancer.
The initial conditions for each simulation take the form (Eq. 3),
for a total of n Gaussians, each with a center μ i , standard deviation σ, and maximum population I 0. Multiple Gaussians may be possible for multiple points of entry from the primary cancer. The number and placement of the initial conditions are approximated and multiple simulation instances performed. Consistent with previous results in modeling gliomas [27], we hold the carrying capacity at K = 108 cells/cm 3, initial condition maxima I 0 = 0.0147 · K, and standard deviation σ = 3 mm.
As with previous studies on GBM growth [19, 27, 28], we assume that the observed velocity represents the asymptotically linear tumor progression of the T1Gd surface (Eq. 4),
Unlike in previous work with brain cancer [4, 28], no relationship between the T2-weighted or FLAIR (fluid attenuation inversion recovery) signals and the population thresholds has been established in peripheral nervous system. This relationship is represented by the ratio λ = D/ρ, or the steepness of a population gradient between low cellular concentrations of the edema response and the high cellular concentration of the T1Gd. To address this, we assume a constant steepness in a numerically stable regime [16] (λ = 2). Choosing this constant value of λ gives numerical stability without the long establishment times of high diffusivity. Assuming limitless growth and the fixed steepness coefficient, the kinetic parameters can be calculated for each subject in terms of the velocity and the steepness (Eqs. 5 and 6),
For each subject, the “growth niduses” were selected based on anatomical points of entry to the lumbosacral plexus, i.e., where the autonomic nerves branch off the plexus.
Atlas creation
Much like atlases of the brain [8, 13], we created a digital atlas of the lumbosacral plexus from the T1Gd scan of a healthy female volunteer. The initial resolution of this scan being 0.7 × 0.7 × 1.0 mm. The segmented lumbosacral plexus is used as the medium in which the simulation evolves. This atlas is spatially refined to represent high resolution of growth. The boundary values apply at the dural edge of each nerve in digital representation.
Model implementation
Solutions to Eq. 1 are generated with a numerical diffusion-reaction solver employing a conjugate gradient method and backwards Euler algorithms written in Python. Each simulation is performed until the T1Gd volumetric radius is 25 mm. Thresholds representing T1Gd scans are tracked both volumetrically and spatially for further analysis.
Rights and permissions
About this article
Cite this article
Jacobs, J.J., Capek, S., Spinner, R.J. et al. Mathematical model of perineural tumor spread: a pilot study. Acta Neurochir 160, 655–661 (2018). https://doi.org/10.1007/s00701-017-3423-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3423-6