Abstract
Background
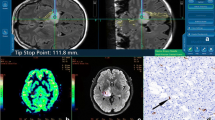
Susceptibility-weighted imaging (SWI) exploits susceptibility differences between tissues to enhance contrast in magnetic resonance imaging to enable the visualization of small blood vessels that are difficult to detect by other contrast agents. This study explored the value of SWI-based planning for neuronavigation-guided deep brain biopsies to reduce the incidence of post-surgical complications.
Methods
The cohort of 84 patients was divided into 41 biopsies performed aided by SWI (SWI group) and 43 biopsies based on conventional T1w-Gd-based imaging (T1w-Gd group). Biopsy targets were determined using magnetic resonance spectroscopy (MRS) before the operation, and the safest trajectory was selected based on preoperative images of blood vessels.
Results
Within 24 h of surgery, there was no radiographically identified bleeding, no blood extravasation and no clinical intracranial hypertension in the SWI group. Only one patient (2.5 %) with basal ganglia lymphoma developed transient hemiparesis after biopsy, who later recovered after undergoing symptomatic treatment. Complication rates in the SWI group were lower than in the T1w-Gd group, where a 7 % morbidity rate was encountered with one patient developing a permanent neurological deficit and two showing biopsy-associated hemorrhages. SWI imaging yielded a better visualization of subcortical vessels and deep-seated brain structures.
Conclusions
SWI-based imaging revealed significantly better visualization of small-caliber vasculature that was not detectable on conventional T1w-Gd imaging, minimizing damage to the brain and reducing postoperative complications. Furthermore, MRS can contribute significantly to target selection to improve the yield of image-guided biopsies.




Similar content being viewed by others
References
Akiyama Y, Miyata K, Harada K, Minamida Y, Nonaka T, Koyanagi I, Asai Y, Houkin K (2009) Susceptibility-weighted magnetic resonance imaging for the detection of cerebral microhemorrhage in patients with traumatic brain injury. Neurol Med Chir (Tokyo) 49(3):97–99
Ayaz M, Boikov AS, Haacke EM, Kido DK, Kirsch WM (2010) Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. J Magn Reson Imaging 31(1):142–148
Barth M, Nöbauer-Huhmann IM, Reichenbach JR, Mlynárik V, Schöggl A, Matula C, Trattnig S (2003) High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla. Investig Radiol 38(7):409–414
Chernov MF, Muragaki Y, Ochiai T, Taira T, Ono Y, Usukura M, Maruyama T, Nakaya K, Nakamura R, Iseki H, Kubo O, Hori T, Takakura K (2009) Spectroscopy-supported frame-based image-guided stereotactic biopsy of parenchymal brain lesions: comparative evaluation of diagnostic yield and diagnostic accuracy. Clin Neurol Neurosurg 111(6):527–535
Essig M, Reichenbach JR, Schad LR, Schoenberg SO, Debus J, Kaiser WA (1999) High-resolution MR venography of cerebral arteriovenous malformations. Magn Reson Imaging 17(10):1417–1425
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18
Frati A, Pichierri A, Bastianello S, Raco A, Santoro A, Esposito V, Giangaspero F, Salvati M (2011) Frameless stereotactic cerebral biopsy: our experience in 296 cases. Stereotact Funct Neurosurg 89(4):234–245
Gilman S (1998) Imaging the brain: first of two parts. N Engl J Med 338(12):812–820
Hall WA (1998) The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 82(9):1749–1755
Hall WA, Truwit CL (2005) 1.5 T: spectroscopy-supported brain biopsy. Neurosurg Clin N Am 16(1):165–172
Haacke EM, DelProposto ZS, Chaturvedi S, Sehgal V, Tenzer M, Neelavalli J, Kido D (2007) Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol 28(2):316–317
Hori M, Ishigame K, Kabasawa H, Kumagai H, Ikenaga S, Shiraga N, Aoki S, Araki T (2010) Precontrast and postcontrast susceptibility-weighted imaging in the assessment of intracranial brain neoplasms at 1.5 T. Jpn J Radiol 28(4):299–304
Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK (2012) Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol 259(7):1426–1432
Kato Y, Sano H, Katada K, Ogura Y, Hayakawa M, Kanaoka N, Kanno T (1999) Application of three-dimensional CT angiography (3D-CTA) to cerebral aneurysms. Surg Neurol 52(2):113–121
Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, Leary MC, Starkman S, Gobin YP, Jahan R, Vespa P, Liebeskind DS, Alger JR, Vinuela F (2002) Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 33(1):95–98
Kim JE, Kim DG, Paek SH, Jung HW (2003) Stereotactic biopsy for intracranial lesions: reliability and its impact on the planning of treatment. Acta Neurochir (Wien) 145(7):547–554
Ku HL, Chi NF (2011) Cerebral lobar microhemorrhages detection by high magnetic field susceptibility weighted image: a potential diagnostic neuroimage technique of Alzheimer’s disease. Med Hypotheses 76(6):840–842
Liu H, Hall WA, Truwit CL (2001) Neuronavigation in interventional MR imaging: Prospective stereotaxy. Neuroimaging Clin N Am 11(4):695–704
Löbel U, Sedlacik H, Sabin ND, Kocak M, Broniscer A, Hillenbrand CM, Patay Z (2010) Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology 52(12):1167–1177
Mittal S, Wu Z, Neelavalli J, Haacke EM (2009) Susceptibility-weighted imaging: technical aspects and clinical application, part 2. AJNR Am J Neuroradiol 30(2):232–252
Mohammed W, Xunning H, Haibin S, Jingzhi M (2013) Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: a review. Cancer Imaging 13(24):186–195
Nishihara M, Takeda N, Harada T, Kidoguchi K, Tatsumi S, Tanaka K, Sasayama T, Kohmura E (2014) Diagnositc yield and morbidity by neuronavigation-guided frameless stereotactic biopsy using magnetic resonance imaging and by frame-based computed tomography-guided stereotactic biopsy. Surg Neurol Int 5(Suppl 8):S421–426
Reichenbach JR, Jonetz-Mentzel L, Fitzek C, Haacke EM, Kido DK, Lee BC, Kaiser WA (2001) High-resolution blood oxygen-level dependent MR venography (HRBV): a new technique. Neuroradiology 43(5):364–369
Robinson RJ, Bhuta S (2011) Susceptibility-weighted imaging of the brain: current utility and potential applications. J Neuroimaging 21(4):e189–204
Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F (2006) Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 48(3):150–159
Schad LR (2001) Improved target volume characterization in stereotactic treatment planning of brain lesions by using high-resolution BOLD MR venography. NMR Biomed 14(7–8):478–483
Schott JM, Reiniger L, Thom M, Holton JL, Grieve J, Brandner S, Warren JD, Revesz T (2010) Brain biopsy in dementia: clinical indications and diagnostic approach. Acta Neuropathol 120(3):327–341
Sehgal V, Delproposto Z, Haddar D (2006) Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 24(1):41–51
Soo TM, Bernstein M, Provias J, Tasker R, Lozano A, Guha A (1995) Failed stereotactic biopsy in a series of 518 cases. Stereotact Funct Neurosurg 64(4):183–196
Stadlbauer A, Moser E, Gruber S, Nimsky C, Fahlbusch R, Ganslandt O (2004) Integration of biochemical images of a tumor into frameless stereotaxy achieved using a magnetic resonance imaging/magnetic resonance spectroscopy hybrid data set. J Neurosurg 101(2):287–294
Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA (2002) Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery 50(4):804–812
Wucliffe ND, Choe J, Holshouser B, Oyoyo UE, Haacke EM, Kido DK (2004) Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: a retrospective study. J Magn Reson Imaging 20(3):372–377
Yamada S, Yamada SM, Nakaguchi H, Murakami M, Hoya K, Matsuno A, Yamazaki K, Ishida Y (2012) Tumefactive multiple sclerosis requiring emergent biopsy and histological investigation to confirm the diagnosis: a case report. J Med Case Rep 6:104
Yoshida Y, Terae S, Kudo K, Tha KK, Imamura M, Miyasaka K (2006) Capillary telangiectasia of the brain stem diagnosed by susceptibility-weighted imaging. J Comput Assist Tomogr 30(6):980–982
Zulfiqar M, Dumrongpisutikul N, Intrapiromkul J, Yousem DM (2012) Detection of intratumoral calcification in oligodendrogliomas by susceptibility-weighted MR imaging. AJNR Am J Neuroradiol 33(5):858–864
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
None.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the ethics committee of the First Affiliated Hospital of China Medical University, Shenyang, China, and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comment
In this manuscript the authors present a novel study on neuronavigation-assisted trajectory planning for deep brain biopsies based on susceptibility-weighted MR imaging. This allows a more precise visualization of the subcortical vasculature and better target selection, especially in combination with MRS, thus resulting in a lower post-biopsy hemorrhage rate. This technique seems valuable to the neurosurgical oncologist, as it decreases the risk of side effects. I encourage further study of this method.
Ekkehard Matthias Kasper
Boston, MA, USA
Rights and permissions
About this article
Cite this article
Wang, X., Li, L., Luo, P. et al. Neuronavigation-assisted trajectory planning for deep brain biopsy with susceptibility-weighted imaging. Acta Neurochir 158, 1355–1362 (2016). https://doi.org/10.1007/s00701-016-2823-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2823-3