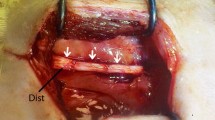
Abstract
Background
Spinal cord injury (SCI) is a complex disease requiring a concerted multi-target approach. The most appropriate combination of therapeutic gene, cellular vehicle, and space filling scaffold still has to be determined. We present an approach that employs syngeneic adipose tissue serving as a three-dimensional biological implant, source of progenitor cells, and delivery system for therapeutic genes. In this pilot experiment, we evaluated the feasibility and short-term effects using gene-activated autologous fat grafts after SCI.
Methods
An experimental SCI model was established in syngeneic Fischer 344 rats by a T9-T10 hemimyelonectomy. Fat tissue was harvested from two donor rats. Animals were divided into four groups and treated with either (i) fat grafts activated by an adenoviral vector carrying the human NT-3 cDNA, (ii) or BDNF, (iii) or with untreated fat grafts or (iv) remained untreated. Animals were euthanized either 7 or 21 days after surgery, and spinal cord tissue was investigated by histological and immunohistochemical methods.
Results
NT-3 and BDNF were produced by gene-activated fat grafts for at least 21 days in vitro and in vivo. Fat tissue grafts remained stable at the site of implantation at 7 days and at 21 days. Neither BDNF-activated nor NT-3-activated fat graft had a detectable limiting effect on the neuronal degeneration. BDNF recruited microglia to perilesional site and attenuated their inflammatory response.
Conclusions
Gene-activated syngeneic fat tissue serves as a three-dimensional biological material delivering therapeutic molecules to the site of SCI over an extended period of time. The BDNF-fat graft attenuated the inflammatory response. Whether these findings translate into functional recovery will require extended observation times.




Similar content being viewed by others
References
Badylak SF, Gilbert TW (2008) Immune response to biologic scaffold materials. Semin Immunol 20:109–116
Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB (1999) NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci 11:3873–3883
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021
Donnelly EM, Strappe PM, McGinley LM, Madigan NN, Geurts E, Rooney GE, Windebank AJ, Fraher J, Dockery P, O’Brien T, McMahon SS (2010) Lentiviral vector-mediated knockdown of the NG2 [corrected] proteoglycan or expression of neurotrophin-3 promotes neurite outgrowth in a cell culture model of the glial scar. J Gene Med 12:863–872
Franz S, Weidner N, Blesch A (2012) Gene therapy approaches to enhancing plasticity and regeneration after spinal cord injury. Exp Neurol 235:62–69
Galli R, Uckermann O, Winterhalder MJ, Sitoci-Ficici KH, Geiger KD, Koch E, Schackert G, Zumbusch A, Steiner G, Kirsch M (2012) Vibrational spectroscopic imaging and multiphoton microscopy of spinal cord injury. Anal Chem 84:8707–8714
Geremia NM, Pettersson LM, Hasmatali JC, Hryciw T, Danielsen N, Schreyer DJ, Verge VM (2010) Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons. Exp Neurol 223:128–142
Gimble JM, Guilak F (2003) Differentiation potential of adipose-derived adult stem (ADAS) cells. Curr Top Dev Biol 58:137–160
Grill RJ, Blesch A, Tuszynski MH (1997) Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol 148:444–452
Gu YL, Yin LW, Zhang Z, Liu J, Liu SJ, Zhang LF, Wang TH (2012) Neurotrophin expressions in neural stem cells grafted acutely to transected spinal cord of adult rats linked to functional improvement. Cell Mol Neurobiol
Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van Der Meide PH, Cullheim S, Olsson T, Piehl F (2000) Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci 20:5283–5291
Harvey AR, Lovett SJ, Majda BT, Yoon JH, Wheeler LP, Hodgetts SI (2014) Neurotrophic factors for spinal cord repair: which, where, how and when to apply, and for what period of time? Brain Res
Hollis ER 2nd, Lu P, Blesch A, Tuszynski MH (2009) IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol 215:53–59
Houweling DA, Lankhorst AJ, Gispen WH, Bar PR, Joosten EAJ (1998) Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol 153:49–59
Hwang DH, Jeong SR, Kim BG (2011) Gene transfer mediated by stem cell grafts to treat CNS injury. Expert Opin Biol Ther 11:1599–1610
Jain A, McKeon RJ, Brady-Kalnay SM, Bellamkonda RV (2011) Sustained delivery of activated Rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PLoS One 6:e16135
Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X (2011) Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience 172:398–405
Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G, Liu X (2010) Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediat Inflamm 2010:372423
Joosten EA, Houweling DA (2004) Local acute application of BDNF in the lesioned spinal cord anti-inflammatory and anti-oxidant effects. Neuroreport 15:1163–1166
Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD (2012) Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res 1438:8–21
Kim H, Tator CH, Shoichet MS (2011) Chitosan implants in the rat spinal cord: biocompatibility and biodegradation. J Biomed Mater Res A 97:395–404
Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W (1997) BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci 17:9583–9595
Koda M, Kamada T, Hashimoto M, Murakami M, Shirasawa H, Sakao S, Ino H, Yoshinaga K, Koshizuka S, Moriya H, Yamazaki M (2007) Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor to bone marrow stromal cells promotes axonal regeneration after transplantation in completely transected adult rat spinal cord. Eur Spine J 16:2206–2214
Kokai LE, Marra K, Rubin JP (2014) Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 163:399–408
Kumamaru H, Saiwai H, Kubota K, Kobayakawa K, Yokota K, Ohkawa Y, Shiba K, Iwamoto Y, Okada S (2013) Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells 31:1535–1547
Le TT, Yue S, Cheng JX (2010) Shedding new light on lipid biology with coherent anti-Stokes Raman scattering microscopy. J Lipid Res 51:3091–3102
Li XL, Zhang W, Zhou X, Wang XY, Zhang HT, Qin DX, Zhang H, Li Q, Li M, Wang TH (2007) Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides 41:135–143
Liu WG, Wang ZY, Huang ZS (2011) Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res 33:686–693
Liu Y, Kim DH, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I (1999) Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci 19:4370–4387
Lu P, Jones LL, Tuszynski MH (2005) BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol 191:344–360
Lu P, Jones LL, Tuszynski MH (2007) Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol 203:8–21
McTigue DM, Horner PJ, Stokes BT, Gage FH (1998) Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci 18:5354–5365
Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB (1998) Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci 10:607–621
Mizoguchi Y, Monji A, Kato T, Seki Y, Gotoh L, Horikawa H, Suzuki SO, Iwaki T, Yonaha M, Hashioka S, Kanba S (2009) Brain-derived neurotrophic factor induces sustained elevation of intracellular Ca2+ in rodent microglia. J Immunol 183:7778–7786
Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, Okano H, Nakamura M (2013) Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain 6:3
Okano T, Nakagawa T, Kita T, Endo T, Ito J (2006) Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol Ther: J Am Soc Gene Ther 14:866–871
Perale G, Rossi F, Santoro M, Peviani M, Papa S, Llupi D, Torriani P, Micotti E, Previdi S, Cervo L, Sundstrom E, Boccaccini AR, Masi M, Forloni G, Veglianese P (2012) Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J Control Release: Off J Control Release Soc 159:271–280
Qin DX, Zou XL, Luo W, Zhang W, Zhang HT, Li XL, Zhang H, Wang XY, Wang TH (2006) Expression of some neurotrophins in the spinal motoneurons after cord hemisection in adult rats. Neurosci Lett 410:222–227
Salgado AJ, Reis RL, Sousa NJ, Gimble JM (2010) Adipose tissue-derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 5:103–110
Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME (1994) Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 367:170–173
Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M (2003) Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol 184:114–130
Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY (2002) Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 99:3024–3029
Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA, Houle JD (2013) Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol 239:91–100
Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A (1996) Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol 137:157–173
Uckermann O, Uhlmann S, Wurm A, Reichenbach A, Wiedemann P, Bringmann A (2005) ADPbetaS evokes microglia activation in the rabbit retina in vivo. Purinergic Signal 1:383–387
Usui N, Watanabe K, Ono K, Tomita K, Tamamaki N, Ikenaka K, Takebayashi H (2012) Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development 139:1125–1132
Van Craenenbroeck K, Vanhoenacker P, Haegeman G (2000) Episomal vectors for gene expression in mammalian cells. Eur J Biochem/FEBS 267:5665–5678
Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F (2011) Bioengineered scaffolds for spinal cord repair. Tissue Eng B Rev 17:177–194
Weishaupt N, Mason AL, Hurd C, May Z, Zmyslowski DC, Galleguillos D, Sipione S, Fouad K (2014) Vector-induced NT-3 expression in rats promotes collateral growth of injured corticospinal tract axons far rostral to a spinal cord injury. Neuroscience 272:65–75
Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB (1995) A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol 134:261–272
Yick LW, Wu WT, So KF, Yip HK (1999) Peripheral nerve graft and neurotrophic factors enhance neuronal survival and expression of nitric oxide synthase in Clarke’s nucleus after hemisection of the spinal cord in adult rat. Exp Neurol 159:131–138
Zhang HT, Luo J, Sui LS, Ma X, Yan ZJ, Lin JH, Wang YS, Chen YZ, Jiang XD, Xu RX (2009) Effects of differentiated versus undifferentiated adipose tissue-derived stromal cell grafts on functional recovery after spinal cord contusion. Cell Mol Neurobiol 29:1283–1292
Zhang X, Xu Y, Wang J, Zhou Q, Pu S, Jiang W, Du D (2012) The effect of intrathecal administration of glial activation inhibitors on dorsal horn BDNF overexpression and hind paw mechanical allodynia in spinal nerve ligated rats. J Neural Transm 119:329–336
Zhou LJ, Yang T, Wei X, Liu Y, Xin WJ, Chen Y, Pang RP, Zang Y, Li YY, Liu XG (2011) Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav Immun 25:322–334
Authors’ contributions
VMB, KHSF, OBB, and MK designed the study. VBM, KHSF, EL, AI, CT, and MS performed experiments. VMB, KHSF, MK, OU, OBB, GS, and HZ analyzed data and wrote the manuscript. MK coordinated the study. All authors commented on and revised the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Animal experiments
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study was approved by the Dresden University of Technology and the federal government of Saxony (24D-9168.11-1-20078), and all procedures involving animals were in accordance with the ethical standards of the Dresden University of Technology and the federal government of Saxony.
Additional information
Volker M. Betz and K. Hakan Sitoci-Ficici contributed equally to this work.
Oliver B. Betz and Matthias Kirsch are co-corresponding authors and contributed equally to this work.
Rights and permissions
About this article
Cite this article
Betz, V.M., Sitoci-Ficici, K.H., Uckermann, O. et al. Gene-activated fat grafts for the repair of spinal cord injury: a pilot study. Acta Neurochir 158, 367–378 (2016). https://doi.org/10.1007/s00701-015-2626-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2626-y