Abstract
Background
In contrast to malignant gliomas, the impact of an early postoperative MRI after surgery of cerebral metastasis is still unclear. The present study analyses early MRI-based postoperative resection controls and incidence of in-brain progression in 116 patients suffering from 130 cerebral metastases.
Methods
The extent of surgical resection was verified by an early postoperative contrast-enhanced 1.5-T MRI within 72 h after surgery of cerebral metastases and correlated with in-brain progression, leptomeningeal carcinomatosis, and progression-free survival.
Results
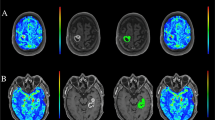
MRI confirmed complete resection was seen in 80 out of 130 metastases (61.5 %). In 24 metastases (18.5 %), no final decision on degree of resection could be made. Residual tumor was seen in 26 cases (20 %). Local in-brain progression was observed in 40 of 130 (30.8 %) cases. The incidence of in-brain progression significantly correlated with dural contact of the metastasis (p < 0.05) and residual tumor on early postoperative MRI (p < 0.0001). The odds ratio for local recurrence with residual tumor is 8.2-fold compared to no residual tumor.
Conclusions
Residual tumor after metastasis extirpation was shown in nearly 20 % of patients by an early postoperative MRI and significantly correlated with local in-brain progression. Furthermore, dural contact of cerebral metastases was identified as a risk factor for local recurrence. Further studies are mandatory to clearly identify the incidence of incomplete resections of cerebral metastases and their oncologic impact. An early postoperative MRI after resection of cerebral metastases is recommended as residual tumor promotes local recurrence.




Similar content being viewed by others
References
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurg 34:45–60, discussion 60–41
Baumert BG, Rutten I, Dehing-Oberije C, Twijnstra A, Dirx MJ, Debougnoux-Huppertz RM, Lambin P, Kubat B (2006) A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 66:187–194
Benveniste RJ, Ferraro N, Tsimpas A (2014) Yield and utility of routine postoperative imaging after resection of brain metastases. J Neurooncol 118:363–367
Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M (2013) Invasion patterns in brain metastases of solid cancers. Neuro-oncol 15:1664–1672
Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, Konig R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36(2), E3. doi:10.3171/2013.11.FOCUS13463
Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, Lopes M, Mitchell MC, Roche S, Muller JC, Bitar A, Sichez JP, van Effenterre R (2003) Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98:764–778
Etminan N, Beseoglu K, Heiroth HJ, Turowski B, Steiger HJ, Hanggi D (2013) Early perfusion computerized tomography imaging as a radiographic surrogate for delayed cerebral ischemia and functional outcome after subarachnoid hemorrhage. Stroke 44:1260–1266
Kamp MA, Dibue M, Niemann L, Reichelt DC, Felsberg J, Steiger HJ, Szelenyi A, Rapp M, Sabel M (2012) Proof of principle: supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochirurg (Wien) 154:1981–1986
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G, Sabel M (2012) 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochirurg (Wien) 154:223–228, discussion 228
10.Kamp MA, Rapp M, Slotty PJ, Turowski B, Sadat H, Smuga M, Dibué-Adjei M, Steiger HJ, Szelényi A, Sabel M (2015) Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir (Wien)PMID 25845550
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141
Lacroix J, Doeberitz MK (2001) Technical aspects of minimal residual disease detection in carcinoma patients. Seminars Surgical Oncol 20:252–264
Neves S, Mazal PR, Wanschitz J, Rudnay AC, Drlicek M, Czech T, Wustinger C, Budka H (2001) Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin Neuropathol 20:38–42
Okuda T, Kataoka K, Yabuuchi T, Yugami H, Kato A (2010) Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. Journal Clin Neurosci 17:118–121
Schebesch KM, Proescholdt M, Hohne J, Hohenberger C, Hansen E, Riemenschneider MJ, Ullrich W, Doenitz C, Schlaier J, Lange M, Brawanski A (2013) Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery–a feasibility study. Acta Neurochir 155:693–699
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Stummer W, Kamp MA (2009) The importance of surgical resection in malignant glioma. Curr Opin Neurol 22:645–649
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Suero Molina EJ, Ardon H, Schroeteler J, Klingenhofer M, Holling M, Wolfer J, Fischer B, Stummer W, Ewelt C (2013) Aquaporin-4 in glioma and metastatic tissues harboring 5-aminolevulinic acid-induced porphyrin fluorescence. Clinical Neurol Neurosurg 115:2075–2081
Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, Fujii K (2007) Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol 24:53–55
Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, Zo JI, Lee SH (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 110:730–736
Conflict of interest
Prof. Sabel is a consultant for Johnson & Johnson Company and Integra Company. Maxine Dibué-Adjei is actually employed by Cyberonics Europe BVBA. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript
Funding
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Presentation at a conference
Annual meeting of the section “Neuro-oncology, Munic Neuro-week 2014, 17.09.2014”
“Early postoperative magnet-resonance tomography after resection of cerebral metastases”
M.A. Kamp, P.J. Slotty, D. Reichelt, H. Sadat, M. Rapp, M. Dibué, H.-J. Steiger, N. Turowski, M. Sabel
Marcel A. Kamp and Marion Rapp contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kamp, M.A., Rapp, M., Bühner, J. et al. Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir 157, 1573–1580 (2015). https://doi.org/10.1007/s00701-015-2479-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2479-4