Abstract
Background
Patients with glioblastoma treated with BCNU wafer implantation for recurrence frequently receive frontline chemoradiotherapy with temozolomide as part of the Stupp protocol. A retrospective investigation was conducted of surgical complications in a cohort of these patients treated at a single institution.
Methods
We searched our institutional database for patients treated between January 2006 and October 2012 who had recurrent glioblastoma previously treated with open surgery followed by the Stupp protocol and then underwent repeat resection with or without BCNU wafers for recurrent disease. Rates of select post-operative complications within 3 months of surgery were estimated.
Results
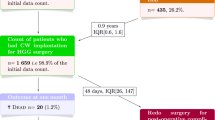
We identified 95 patients with glioblastoma who underwent resection followed by the Stupp protocol as frontline treatment. At disease recurrence (first and second recurrence), 63 patients underwent repeat resection with BCNU wafer implantation and 32 without implantation. Generally, BCNU wafer use was associated with minor to moderate increases in rates of select complications versus non-implantation—wound healing abnormalities (14.2 vs. 6.2 %), cerebrospinal fluid leak (7.9 vs. 3.1 %), hydrocephalus requiring ventriculoperitoneal shunt (6.3 vs. 9.3 %), chemical meningitis (3.1 vs. 0 %), cerebral infections (3.1 vs. 0 %), cyst formation (3.1 vs. 3.1 %), cerebral edema (4.7 vs. 0 %), and empyema formations (1.5 vs. 0 %). Performance status was well maintained post-operatively in both groups. Median progression-free survival from the time of first recurrence was 6.0 and 5.0 months, respectively.
Conclusions
The use of the Stupp protocol as frontline therapy in patients with glioblastoma does not preclude the use of BCNU wafers at the time of progression.


Similar content being viewed by others
References
Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H (2008) Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol 15:2887–2893
Balmaceda C, Peereboom D, Pannullo S, Cheung YK, Fisher PG, Alavi J, Sisti M, Chen J, Fine RL (2008) Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer 112:1139–1146
Balossier A, Dorner L, Emery E, Heese O, Mehdorn HM, Menei P, Singh J (2010) Incorporating BCNU wafers into malignant glioma treatment: European case studies. Clin Drug Investig 30:195–204
Barker FG 2nd (1994) Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery 35:484–490
Barker FG 2nd (2007) Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery 60:887–894
Bock HC, Cohnen J, Keric N, Kantelhardt SR, Giese A (2011) Occlusion of surgical opening of the ventricular system with fibrinogen-coated collagen fleece: a case collection study. Acta Neurochir (Wien) 153:533–539
Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A (2010) First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 33:441–449
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet 345:1008–1012
Della Puppa A, Rossetto M, Ciccarino P, Denaro L, Rotilio A, d'Avella D, Scienza R (2011) Carmustine wafer implantation when surgical cavity is communicating with cerebral ventricles: technical considerations on a clinical series. World Neurosurg 76:156–159
Deorah S, Lynch CF, Sibenaller ZA, Ryken TC (2006) Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus 20:E1
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Dixit S, Hingorani M, Achawal S, Scott I (2011) The sequential use of carmustine wafers (Gliadel(R)) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg 25:459–469
Engelhard HH (2000) Tumor bed cyst formation after BCNU wafer implantation: report of two cases. Surg Neurol 53:220–224
Engelhard HH, Maher de Leon M, Rozental J (1998) Tumor bed cyst formation after BCNU wafer implantation. Annual Meeting of the Congress of Neurological Surgeons, Seattle
Fadul CE, Wen PY, Kim L, Olson JJ (2008) Cytotoxic chemotherapeutic management of newly diagnosed glioblastoma multiforme. J Neurooncol 89:339–357
Fleming AB, Saltzman WM (2002) Pharmacokinetics of the carmustine implant. Clin Pharmacokinet 41:403–419
Gallego JM, Barcia JA, Barcia-Marino C (2007) Fatal outcome related to carmustine implants in glioblastoma multiforme. Acta Neurochir 149:261–265
Giese A, Bock HC, Kantelhardt SR, Rohde V (2010) Risk management in the treatment of malignant gliomas with BCNU wafer implants. Cen Eur Neurosurg 71:199–206
Giese A, Kucinski T, Knopp U, Goldbrunner R, Hamel W, Mehdorn HM, Tonn JC, Hilt D, Westphal M (2004) Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol 66:351–360
Giese A, Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39:235–250
Gottfried ON, Deogaonkar M, Way DL, Hamilton AJ (2000) Postoperative cerebral edema after intracavitary implantation of Gliadel(R) wafers for treatment of malignant gliomas [abstract]. J Invest Med 48:79
Gururangan S, Cokgor L, Rich JN, Edwards S, Affronti ML, Quinn JA, Herndon JE 2nd, Provenzale JM, McLendon RE, Tourt-Uhlig S, Sampson JH, Stafford-Fox V, Zaknoen S, Early M, Friedman AH, Friedman HS (2001) Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro Oncol 3:246–250
Gutenberg A, Lumenta CB, Braunsdorf WE, Sabel M, Mehdorn HM, Westphal M, Giese A (2013) The combination of carmustine wafers and temozolomide for the treatment of malignant gliomas. A comprehensive review of the rationale and clinical experience. J Neurooncol 113:163–174
Kleinberg LR, Weingart J, Burger P, Carson K, Grossman SA, Li K, Olivi A, Wharam MD, Brem H (2004) Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest 22:1–9
La Rocca RV, Vitaz TW, Villaneuver W, Hodes J, Cervera A, New P, Litofsky NS (2008) A Phase 2 Study of Multimodal Therapy With Surgery, Carmustine (BCNU) Wafer, Radiation, and Temozolomide in Patients With Newly Diagnosed Supratentorial Malignant Glioma. Abstract and poster presented at the 8th Meeting of the European Association of Neurooncology (EANO), September 12–14, Barcelona
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A (2009) Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110:583–588
Menei P, Metellus P, Parot-Schinkel E, Loiseau H, Capelle L, Jacquet G, Guyotat J (2010) Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol 17:1740–1746
Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher-Schroder E, Mittermeyer S, Westphal M, Sampson JH, Vogelbaum MA, Croteau D, Chang SM (2011) Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101:267–277
National Comprehensive Cancer Network NCCN (2012) Clinical Practice Guidelines in Oncology. Central nervous system cancer. Version 1
Noel G, Schott R, Froelich S, Gaub MP, Boyer P, Fischer-Lokou D, Dufour P, Kehrli P, Maitrot D (2012) Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior Gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys 82:749–755
Pan E, Mitchell SB, Tsai JS (2008) A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol 88:353–357
Perry JR, Belanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28:2051–2057
Sabel M, Giese A (2008) Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin 24:3239–3257
Salvati M, D'Elia A, Frati A, Brogna C, Santoro A, Delfini R (2011) Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high-grade gliomas at first diagnosis. J Neurosurg Sci 55:1–6
Sampath P, Brem H (1998) Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control 5:130–137
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C, Franz K, Pietsch T (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Tonn JC, Brada M, Pentheroudakis G (2010) High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v190–v193
Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M, Schiff D (1999) Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery 45:17–22
Tamargo RJ, Epstein JI, Reinhard CS, Chasin M, Brem H (1989) Brain biocompatibility of a biodegradable, controlled-release polymer in rats. J Biomed Mater Res 23:253–266
Tran B, Rosenthal MA (2010) Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci 17:417–421
Ulmer S, Spalek K, Nabavi A, Schultka S, Mehdorn HM, Kesari S, Dorner L (2012) Temporal changes in magnetic resonance imaging characteristics of Gliadel wafers and of the adjacent brain parenchyma. Neuro Oncol 14:482–490
Weber EL, Goebel EA (2005) Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol 7:84–89
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5:79–88
Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, Meyermann R, Reifenberger G, Weller M, Wick W (2007) Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25:3357–3361
Acknowledgments
Writing and editorial assistance was provided by Michael Raffin (Fishawack Communications), and was financially supported by Archimedes Pharma.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samis Zella, M.A., Wallocha, M., Slotty, P.J. et al. Evaluation of post-operative complications associated with repeat resection and BCNU wafer implantation in recurrent glioblastoma. Acta Neurochir 156, 313–323 (2014). https://doi.org/10.1007/s00701-013-1931-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1931-6