Abstract
Background
Wnt proteins are bifunctional axon guidance molecules, several of which appear to mediate guidance of corticospinal tract axons along the spinal cord. Here, we studied increasing effect on regeneration by Wnt-containing alginate scaffolds on spinal cord injury (SCI).
Methods
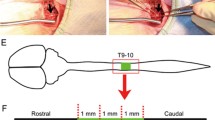
A total of 32 rats were injured at the T7–8 level with an NYU impactor. According to transplantation materials, rats were classified into four groups: a Wnt3a-secreting fibroblast transplantation group (Wnt group, n = 8), a Wnt3a-secreting fibroblast with alginate transplantation group (Wnt + alginate group, n = 8), an alginate transplantation group (alginate group, n = 8), and a contusion-only group (sham group, n = 8). Behavioral tests were performed on the first, second, and third days after injury, and then weekly for 8 weeks. Five of the eight rats from each group were selected for manganese-enhanced magnetic resonance imaging (ME-MRI). Two rats from each group were examined for GAP43 and MAP2 expression using monoclonal and polyclonal primary antibodies, respectively.
Results
Seven weeks after transplantation (8 weeks after SCI), Wnt + alginate group rats achieved an average Basso–Beattie–Bresnahan locomotor score of 19.0, which was significantly higher than that of other groups. ME-MRI at 8 weeks after SCI revealed significantly higher relative signal intensities in the Wnt + alginate group. Gap43 and Map2 immunostaining, showed strong positive in the Wnt + alginate group.
Conclusion
The Wnt + alginate complex exerted significantly enhanced recovery in a rat SCI model compared to alginate or Wnt3a alone. These results suggest that alginate scaffolds facilitate the regeneration of axon working with Wnt3a protein that promotes regeneration of the injured spinal cord.







Similar content being viewed by others
References
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20(2):84–91
Bilgen M, Dancause N, Al-Hafez B, He YY, Malone TM (2005) Manganese-enhanced MRI of rat spinal cord injury. Magn Reson Imaging 23(7):829–832
Burry RW, Lah JJ, Hayes DM (1992) GAP-43 distribution is correlated with development of growth cones and presynaptic terminals. J Neurocytol 21(6):413–425
Chen BK, Knight AM, de Ruiter GC, Spinner RJ, Yaszemski MJ, Currier BL, Windebank AJ (2009) Axon regeneration through scaffold into distal spinal cord after transection. J Neurotrauma 26(10):1759–1771
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297(5580):365–369
David MD, Canti C, Herreros J (2010) Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res 88(14):3011–3023
Frey D, Laux T, Xu L, Schneider C, Caroni P (2000) Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J Cell Biol 149(7):1443–1454
Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116(6):769–778
Goslin K, Schreyer DJ, Skene JH, Banker G (1990) Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci 10(2):588–602
Gulacsi AA, Anderson SA (2008) Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci 11(12):1383–1391
Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y (2004) The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131(12):2791–2801
Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115(Pt 21):3977–3978
Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S (1997) Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389(6654):966–970
Im J, Kim H, Kim S, Jho EH (2007) Wnt/beta-catenin signaling regulates expression of PRDC, an antagonist of the BMP-4 signaling pathway. Biochem Biophys Res Commun 354(1):296–301
Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, Ide C (2004) Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng 10(3–4):493–504
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437(7063):1370–1375
Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129(9):2087–2098
Michaelidis TM, Lie DC (2008) Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res 331(1):193–210
Murashov AK, Pak ES, Hendricks WA, Owensby JP, Sierpinski PL, Tatko LM, Fletcher PL (2005) Directed differentiation of embryonic stem cells into dorsal interneurons. FASEB J 19(2):252–254
Namgung U, Routtenberg A (2000) Transcriptional and post-transcriptional regulation of a brain growth protein: regional differentiation and regeneration induction of GAP-43. Eur J Neurosci 12(9):3124–3136
Neve RL, Coopersmith R, McPhie DL, Santeufemio C, Pratt KG, Murphy CJ, Lynn SD (1998) The neuronal growth-associated protein GAP-43 interacts with rabaptin-5 and participates in endocytosis. J Neurosci 18(19):7757–7767
Oestreicher AB, De Graan PN, Gispen WH, Verhaagen J, Schrama LH (1997) B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog Neurobiol 53(6):627–686
Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA (2004) Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131(15):3545–3557
Parr BA, Shea MJ, Vassileva G, McMahon AP (1993) Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119(1):247–261
Sousa KM, Villaescusa JC, Cajanek L, Ondr JK, Castelo-Branco G, Hofstra W, Bryja V, Palmberg C, Bergman T, Wainwright B, Lang RA, Arenas E (2010) Wnt2 regulates progenitor proliferation in the developing ventral midbrain. J Biol Chem 285(10):7246–7253
Stieltjes B, Klussmann S, Bock M, Umathum R, Mangalathu J, Letellier E, Rittgen W, Edler L, Krammer PH, Kauczor HU, Martin-Villalba A, Essig M (2006) Manganese-enhanced magnetic resonance imaging for in vivo assessment of damage and functional improvement following spinal cord injury in mice. Magn Reson Med 55(5):1124–1131
Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR (2011) Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 153(5):1003–1010
Suzuki Y, Kitaura M, Wu S, Kataoka K, Suzuki K, Endo K, Nishimura Y, Ide C (2002) Electrophysiological and horseradish peroxidase-tracing studies of nerve regeneration through alginate-filled gap in adult rat spinal cord. Neurosci Lett 318(3):121–124
Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F (2011) Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev 17(3):177–194
Woodhead GJ, Mutch CA, Olson EC, Chenn A (2006) Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci 26(48):12620–12630
Yun S, Rim Y, Jho EH (2007) Induced expression of the transcription of tropomodulin 1 by Wnt5a and characterization of the tropomodulin 1 promoter. Biochem Biophys Res Commun 363(3):727–732
Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, Birchmeier W, Birchmeier C (2003) Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 258(2):406–418
Acknowledgment
This work was supported by an Asan Life Science Institute Grant (12-176) from the Asan Medical Center, Seoul, Korea, and by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0000447).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin Hoon Park and Joongkee Min contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, J.H., Min, J., Baek, S.R. et al. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir 155, 809–816 (2013). https://doi.org/10.1007/s00701-013-1663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1663-7