Abstract
Background
The aim of this study was to evaluate gross and microscopic anatomical features of the vestibulocochlear nerve or eighth cranial nerve (CNVIII) from fresh cadavers, especially the nerve’s central myelin portion (CMP) and transitional zone (TZ), and to consider any pathological implications.
Methods
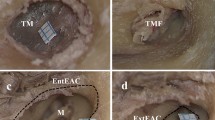
Six fresh cadavers were used to examine the CNVIII. Its cisternal length from brainstem to internal auditory meatus was measured. Longitudinal sections were stained to make following measurements: the diameter where the nerve enters the brainstem, the diameter where the TZ begins, the distance to the most distal part of TZ from the brainstem, and the depth of the TZ. The volume of the CMP was calculated as well.
Results
The cisternal length of ten CNVIIIs measured between 14.2 and 19.2 mm (16.48 ±1.78 mm). The thickness where the CNVIII enters the brainstem was between 1.21 and 3.16 mm (2.31 ± 0.68 mm); the thickness where the TZ begins was between 1.07 and 2.21 mm (1.44 ± 0.38 mm); the distance of the most distal part of the TZ from the brainstem was between 9.28 and 13.84 mm (11.50 ± 1.56 mm); the depth of the TZ was between 0.56 and 1.28 mm (0.81 ± 0.27 mm). The volume of the CMP was between 17.34 and 53.87 mm3 (33.98 ± 13.74 mm3). The measurements were compared to trigeminal, facial, glossopharyngeal and vagus nerves. CNVIII was the nerve with the longest CMP.
Conclusions
The measurements showed that the CMP of CNVIII was very long. The implication of this length in the dysfunctional syndromes of this nerve, its propensity to harbor schwannomas, which most frequently arise at the porus of the auditory meatus, and the vulnerability to damages are discussed.






Similar content being viewed by others
References
Bridger MW, Farkashidy J (1980) The distribution of neuroglia and Schwann cells in the 8th nerve of man. J Laryngol Otol 94:1353–1362
De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L (2002) Is the root entry/exit zone important in microvascular compression syndromes ? Neurosurgery 51:427–434
Escourolle R, Poirier J (1971) Electron microscope study of nervous system tumors. Neurochirurgie (French) 17:25–49
Foncin JF, Sterkers JM, Perre J, Corlieu P (1979) The origin of acoustic neurionoma. An ultrastructural study of operated neurinoma incipiens. Ann Otolaryngol Chir Cervicofac (French) 96:11–22
Guclu B, Meyronet D, Simon E, Streichenberger N, Sindou M, Mertens P (2009) Structural anatomy of cranial nerves (V, VII, VIII, IX, X). Neurochirurgie (French) 55:92–98
Guclu B, Sindou M, Meyronet D, Streichenberger N, Simon E, Mertens P (2011) Cranial nerve vascular compression syndromes of the trigeminal, facial and vago-glossopharyngeal nerves: comparative anatomical study of the central myelin portion and transitional zone; correlations with incidences of corresponding hyperactive dysfunctional syndromes. Acta Neurochir (Wien) 153:2365–2375
Guevara N, Deveze A, Buza V, Laffont B, Magnan J (2008) Microvascular decompression of cochlear nerve for tinnitus incapacity: pre-surgical data, surgical analyses and long-term follow-up of 15 patients. Eur Arch Otorhinolaryngol 265:397–401
Jannetta PJ (1975) Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg Forum 26:467–469
Jannetta PJ (1997) Selection criteria for the treatment of cranial rhizopathies by microvascular decompression (honored guest lecture). Clin Neurosurg 44:69–77
Jannetta PJ, Møller MB, Møller AR (1984) Disabling positional vertigo. N Engl J Med 310:1700–1705
Møller AR (1984) Pathophysiology of tinnitus. Ann Otol Rhinol Laryngol 93:39–44
Møller AR (2006) Neural Plasticity and Disorders of the Nervous System, Cambridge University Press
Møller MB, Møller AR, Jannetta PJ, Jho HD (1993) Vascular decompression surgery for severe tinnitus: selection criteria and results. Laryngoscope 103:421–427
Møller MB, Møller AR, Jannetta PJ, Jho HD, Sekhar LN (1993) Microvascular decompression of the eighth nerve in patients with disabling positional vertigo: selection criteria and operative results in 207 patients. Acta Neurochir (Wien) 125:75–82
Murray MR, Stout AP, Bradley CF (1940) Schwann cell versus fibroblast as the origin of the specific nerve sheath tumor: Observations upon normal nerve sheaths and neurilemomas in vitro. Am J Pathol 16:41–60
Neely JG, Britton BH, Greenberg SD (1976) Microscopic characteristics of the acoustic tumor in relationship of its nerve of origin. Laryngoscope 86:984–991
Obersteiner H, Redlich E (1894) Uber Wesen und Pathogenese der Tabischen Hinterstrangsdegeneration. Arb Neurol Inst, Wien Univ 1–3:158–172
Pellet W, Roche PH (2004) Microsurgery of vestibular schwannoma: persisting questions. Neurochirurgie (French) 50:195–243
Pirsig W, Eckermeier L, Mueller D (1979) As to the origin of vestibular schwannomas. Seminar on Diagnostic and Management of Acoustic Tumors. Ear Research Institute. Los Angeles, 1979. In: House WF (ed) Acoustic Tumors, vol 1. University Park Press, Baltimore, pp 52–55
Rubinstein D, Sandberg EJ, Cajade-Law AG (1996) Anatomy of the facial and vestibulocochlear nerves in the internal auditory canal. AJNR Am J Neuroradiol 17:1099–1105
Ryu H, Yamamoto S, Sugiyama K, Nozue M (1998) Neurovascular compression syndrome of the eighth cranial nerve. What are the most reliable diagnostic signs? Acta Neurochir (Wien) 140:1279–1286
Schefter RP, Harner SG (1986) Histologic study of the vestibulocochlear nerve. Ann Otol Rhinol Laryngol 95:146–150
Skinner HA (1929) The origin of acoustic nerve tumours. Br J Surg 16:440–463
Skinner HA (1931) Some histologic features of the cranial nerves. Archs Neurol Psychiat 25:356–372
Tarlov IM (1937) Structure of the nerve root. I. Nature of the junction between the central and the peripheral nervous system. Archs Neurol Psychiat 37:555–583
Tarlov IM (1937) Structure of the nerve root. II. Differentiation of sensory from motor roots; observations on identification of function in roots of mixed cranial nerves. Archs Neurol Psychiat 37:1338–1355
Vasama JP, Moller MB, Moller AR (1998) Microvascular decompression of the cochlear nerve in patients with severe tinnitus. Preoperative findings and operative outcome in 22 patients. Neurol Res 20:242–248
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Guclu and colleagues from the University of Lyon report the results of a microscopic anatomy study on the length of central myelin of the VIIIth cranial nerve. This study completes a series of volumetric analyses of the central myelin of the cranial nerves and confirms that the central myelin portion of the vestibulocochlear nerve is the longest.
The authors suggest that this feature may predispose the VIIIth cranial nerve to dysfunctional syndromes, such as positional vertigo and tinnitus, and its peculiar vulnerability to mechanical injuries. Also, the authors suggest its role in the susceptibility of the vestibulocochlear nerve to the development of schwannomas. The hypothesis is attractive, but it needs to be verified by more comprehensive studies.
One point, however, deserves attention: the embryologic origin of the nerve and its possible influence on the development of vestibular schwannomas. This and other well-known studies showed that schwannomas develop at the glio-schwannian junction. Here, in particular circumstances, Schwann cells develop some potential to generate proliferative tumor cells. Therefore, we can infer that in this small area, there are environmental factors, including an irregular glio-schwannian interaction, contributing to the development of tumors.
Alfredo Conti Messina, Italy
Rights and permissions
About this article
Cite this article
Guclu, B., Sindou, M., Meyronet, D. et al. Anatomical study of the central myelin portion and transitional zone of the vestibulocochlear nerve. Acta Neurochir 154, 2277–2283 (2012). https://doi.org/10.1007/s00701-012-1479-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1479-x