Abstract
Background
Wound-healing problems in the neurosurgical patient can be particularly bothersome, owing to various specific risk factors involved. These may vary from simple wound dehiscence to complex multi-layer defects with cerebrospinal fluid (CSF) leakage and contamination. The latter is quite rare in practice and requires an individually titrated reconstruction strategy. The objective is to retrospectively analyze neurosurgical patients with complex, recalcitrant wound-healing problems we had treated in our department, attempt to develop a grading system based on the risk factors specific to our specialty and adapt a surgical reconstruction algorithm.
Methods
During an 11-year period, 49 patients were identified to have had complex, recalcitrant wound-healing problems involving the cranial vault (n = 43) and the skull base (n = 6) that required an adapted surgical wound-management strategy. The etiologies of wound healing problems were aftermaths of surgical treatment of: (1) brain tumors (nine cases), (2) aneurysm clipping (ten cases), (3) trauma (27 patients), and (4) congenital malformations (three patients). Local rotational advancement flaps were performed in 18 patients and free microvascular tissue transfer was performed in 37 cases.
Results
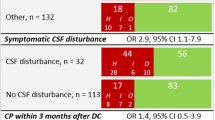
Major risk factors leading to recalcitrant wound healing problems in the presented group were: prolonged angiographic interventions (20%), ongoing chemotherapy or radiotherapy (47%), prolonged cortisone application (51%), CSF leak (76%) and, above all, multiple failed attempts at wound closure (94%). Stable long-term wound healing was achieved in all patients using vascularized tissue coverage. A ternary grading system was developed based on various risk factors in the presented cohort. Accordingly, the algorithm for reconstruction in neurosurgical patients was adapted.
Conclusions
Primary disease, treatment history, and distorted anatomical structures are major concerns in the management of complex wound-healing problems in neurosurgical patients. The higher the risk factors involved, the more complex is the surgical strategy. Free microvascular tissue transfer offers stable long-term results in recalcitrant cases. However, this may be indicated only in patients with a good prognosis of the underlying disease.







Similar content being viewed by others
References
Editorial Board of Plastic and Reconstructive Surgery (2008) Management of a complex scalp defect. Plast Reconstr Surg 122:623–625
Allen MBJ (1990) Complications, their prevention and treatment. In: Preoperative evaluation, 3rd edn. Saunders, Phildelphia
Antonyshyn O, Colcleugh RG, Anderson C (1987) Growth potential in onlay bone grafts: a comparison of vascularized and free calvarial bone and suture bone grafts. Plast Reconstr Surg 79:12–23
Antonyshyn O, Colcleugh RG, Anderson C (1987) Growth potential in suture bone inlay grafts: a comparison of vascularized and free calvarial bone grafts. Plast Reconstr Surg 79:1–11
Babuccu O, Kalayci M, Peksoy I, Kargi E, Cagavi F, Numanoğlu G (2004) Effect of cerebrospinal fluid leakage on wound healing in flap surgery: histological evaluation. Pediatr Neurosurg 40:101–106
Balch RE (1967) Wound infections complicating neurosurgical procedures. J Neurosurg 26:41–45
Barrow DL, Nahai F, Tindall GT (1984) The use of greater omentum vascularized free flaps for neurosurgical disorders requiring reconstruction. J Neurosurg 60:305–311
Bernstein EF, Sullivan FJ, Mitchell JB, Salomon GD, Glatstein E (1993) Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg 20:435–453
Blomstedt GC (1992) Craniotomy infections. Neurosurg Clin N Am 3:375–385
Blomstedt GC (1985) Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir (Wien) 78:81–90
Bruce JN, Bruce SS (2003) Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg 98:1203–1207
Calderon W, Chang N, Mathes SJ (1986) Comparison of the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg 77:785–794
Chicarilli ZN, Ariyan S, Cuono CB (1986) Single-stage repair of complex scalp and cranial defects with the free radial forearm flap. Plast Reconstr Surg 77:577–585
David DJ, Cooter RD (1987) Craniofacial infection in 10 years of transcranial surgery. Plast Reconstr Surg 80:213–225
Disa JJ, Smith AW, Bilsky MH (2001) Management of radiated reoperative wounds of the cervicothoracic spine: the role of the trapezius turnover flap. Ann Plast Surg 47:394–397
Furnas H, Lineaweaver WC, Alpert BS, Buncke HJ (1990) Scalp reconstruction by microvascular free tissue transfer. Ann Plast Surg 24:431–444
Gahhos FN, Ariyan S, Cuono CB, Arons MS (1985) Burn wound excision and local flap closure. Ann Plast Surg 14:535–540
Ghogawala Z, Mansfield FL, Borges LF (2001) Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine (Phila Pa 1976) 26:818–824
Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD (2004) Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg 100:163–168
Hierner R, van Loon J, Goffin J, van Calenbergh F (2007) Free latissimus dorsi flap transfer for subtotal scalp and cranium defect reconstruction: report of 7 cases. Microsurgery 27:425–428
Hussussian CJ, Reece GP (2002) Microsurgical scalp reconstruction in the patient with cancer. Plast Reconstr Surg 109:1828–1834
Iida H (2003) The advantage of the anterolateral thigh flap for reconstruction of the anterior skull base defect in recurrent cases. Plast Reconstr Surg 112:703–704
Ioannides C, Fossion E, McGrouther AD (1999) Reconstruction for large defects of the scalp and cranium. J Craniomaxillofac Surg 27:145–152
Izquierdo R, Leonetti JP, Origitano TC, al-Mefty O, Anderson DE, Reichman OH (1993) Refinements using free-tissue transfer for complex cranial base reconstruction. Plast Reconstr Surg 92:567–574
Izquierdo R, Origitano TC, al-Mefty O, Leonetti JP, Anderson DE, Reichman OH (1993) Use of vascularized fat from the rectus abdominis myocutaneous free flap territory to seal the dura of basicranial tumor resections. Neurosurgery 32:192–196
Korinek AM (1997) Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery 41:1073–1079
Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, Puybasset L (2005) Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg 19:155–162
Koshima I, Yamamoto H, Hosoda M, Moriguchi T, Orita Y, Nagayama H (1993) Free combined composite flaps using the lateral circumflex femoral system for repair of massive defects of the head and neck regions: an introduction to the chimeric flap principle. Plast Reconstr Surg 92:411–420
Lara WC, Schweitzer J, Lewis RP, Odum BC, Edlich RF, Gampper TJ (1998) Technical considerations in the use of polymethylmethacrylate in cranioplasty. J Long Term Eff Med Implants 8:43–53
Larsson A, Engstrom M, Uusijarvi J, Kihlström L, Lind F, Mathiesen T (2002) Hyperbaric oxygen treatment of postoperative neurosurgical infections. Neurosurgery 50:287–295
Lee B, Bickel K, Levin S (1999) Microsurgical reconstruction of extensive scalp defects. J Reconstr Microsurg 15:255–262
Lee SC, Wu CT, Lee ST, Chen PJ (2009) Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci 16:56–63
Leedy JE, Janis JE, Rohrich RJ (2005) Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg 116:54e–72e
McCombe D, Donato R, Hofer SO, Morrison W (2002) Free flaps in the treatment of locally advanced malignancy of the scalp and forehead. Ann Plast Surg 48:600–606
Mehrara BJ, Disa JJ, Pusic A (2006) Scalp reconstruction. J Surg Oncol 94:504–508
Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V (2003) Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg 14:144–153
Moyer JS, Chepeha DB, Teknos TN (2004) Contemporary skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg 12:294–299
Murphy RC, Robson MC, Heggers JP, Kadowaki M (1986) The effect of microbial contamination on musculocutaneous and random flaps. J Surg Res 41:75–80
Newman MI, Hanasono MM, Disa JJ, Cordeiro PG, Mehrara BJ (2004) Scalp reconstruction: a 15-year experience. Ann Plast Surg 52:501–506
Oishi SN, Luce EA (1995) The difficult scalp and skull wound. Clin Plast Surg 22:51–59
Pennington DG, Stern HS, Lee KK (1989) Free-flap reconstruction of large defects of the scalp and calvarium. Plast Reconstr Surg 83:655–661
Rasmussen S, Ohrstrom JK, Westergaard L, Kosteljanetz M (1990) Post-operative infections of osteoplastic compared with free bone flaps. Br J Neurosurg 4:493–495
Serletti JM (2008) Management of a complex scalp defect. Plast Reconstr Surg 122:626–629, Discussion
Shen YM, Shen ZY (2003) Greater omentum in reconstruction of refractory wounds. Chin J Traumatol 6:81–85
Stark AM, Eichmann T, Mehdorn HM (2003) Skull metastases: clinical features, differential diagnosis, and review of the literature. Surg Neurol 60:219–225
Stula D (1982) The problem of the “sinking skin-flap syndrome” in cranioplasty. J Maxillofac Surg 10:142–145
Tanaka Y, Matsumoto K, Song S, Tajima S, Ohmura T (1997) Reconstruction of a cranial bone defect with hydroxyapatite and free flap transfer. J Craniofac Surg 8:141–145
Tenney JH, Vlahov D, Salcman M, Ducker TB (1985) Wide variation in risk of wound infection following clean neurosurgery. Implications for perioperative antibiotic prophylaxis J Neurosurg 62:243–247
Tessier P (1982) Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg 9:531–538
Tokarek R, Bernstein EF, Sullivan F, Uitto J, Mitchell JB (1994) Effect of therapeutic radiation on wound healing. Clin Dermatol 12:57–70
Vitaz TW, Oishi M, Welch WC, Gerszten PC, Disa JJ, Bilsky MH (2004) Rotational and transpositional flaps for the treatment of spinal wound dehiscence and infections in patient populations with degenerative and oncological disease. J Neurosurg 100:46–51
Wang HT, Erdmann D, Olbrich KC, Friedman AH, Levin LS, Zenn MR (2007) Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg 119:865–872
Weinstein MA, McCabe JP, Cammisa FP Jr (2000) Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 13:422–426
Wilhelmi BJ, Snyder N, Colquhoun T, Hadjipavlou A, Phillips LG (2000) Bipedicle paraspinous muscle flaps for spinal wound closure: an anatomic and clinical study. Plast Reconstr Surg 106:1305–1311
Wimmer C, Gluch H, Franzreb M, Ogon M (1998) Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord 11:124–128
Wright RL (1966) A survey of possible etiologic agents in postoperative craniotomy infections. J Neurosurg 25:125–132
Yoshioka N, Haraoka G, Muraoka M, Tominaga S (1996) Single stage reconstruction of scalp and skull using free muscle flap and titanium mesh in patients with epidural infection. J Craniomaxillofac Surg 24:118–121
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The Authors have expanded their considerable surgical experience on complex wound healing management into the form of this valuable scientific report. The issue is important and rather underscored by the current literature. The Author's surgical techniques, description of illustrative cases, and conclusions are clearly presented and convincingly discussed. I am less enthusiastic concerning the grading and management algorithms, which, although logical and condivisible, are more reflecting expert's opinions rather than evidence-based scientific conclusions.
Domenico d'Avella
Padova, Italy
During a three-month service adjacent to the carnage of WW1, Harvey Cushing reduced the ‘accepted’ mortality of 50% in skull injuries with dural penetration to 29% in his 133 patients by meticulous technique and closure (1), concluding that ‘success in closing defects is the offspring of ingenuity and experience’.
The authors feel that wound healing problems are underreported in neurosurgery. Actually, a great majority of neurosurgical wounds seem to heal per primam intentionem in modern neurosurgery, according to the PubMed articles from the last 10 years: wound healing and neurosurgery 670 – neuroendoscopy 693 – acoustic neurinomas 2104 – meningiomas 5435 – intracranial aneurysms 7522 – glioblastomas 9082. Instead, the EANS Training Course programs witness that the problems of wound healing and their prevention and management are by far underdiscussed with the residents – the ones who will close most of the wounds per primam and even per secundam. And skull base tumours, aneurysms and AVMs of gargantuan sizes are by far overdiscussed for population based practices.
The authors report on adapted surgical wound management strategies in 49 patients with complex, recalcitrant (Thesaurus: disobedient, uncontrollable) wound healing problems involving the cranial vault (n = 43) and the skull base (n = 6). On-going chemo or radiotherapy (47%), prolonged cortisone application (51%), CSF leak (76%) and multiple failed attempts at wound closure (94%) were major risk factors. Local rotational advancement flaps were performed in 18 and free microvascular tissue transfer in 37. The authors conclude the obvious - ‘free microvascular tissue transfer is the preferred choice to reconstruct large and complex wound healing problems’.
This is highly recommendable reading for any neurosurgeon and for residents in particular. Furthermore, all operations here were performed by the first author, trained in plastic & reconstructive microsurgery as well as in neurosurgery (!). Which comes to the contents of our residency programs - which (micro)surgical skills to obtain from outside the traditional scope of neurosurgery ?
Juha E Jääskeläinen
Kuopio, Finland
1. Cushing H (1918) Notes on penetrating wounds of the brain. Br Med J 23(2982):221-226
A portion of this work was presented in poster form at the 62nd Annual Meeting of the German Neurosurgical Society, Hamburg, May 9th, 2011
Rights and permissions
About this article
Cite this article
Krishnan, K.G., Müller, A., Hong, B. et al. Complex wound-healing problems in neurosurgical patients: risk factors, grading and treatment strategy. Acta Neurochir 154, 541–554 (2012). https://doi.org/10.1007/s00701-011-1221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1221-0