Abstract
Objective
Epidemiological studies indicate a link between low-dose irradiation (<10,000 mGy) to the head and the local occurrence of tumors after decades of delay. Comparable radiation doses can be reached during neuro-endovascular procedures (NEP), but the incidence of similar exposures has not been completely delineated. We compared the levels of radiation to the head measured during NEP to those reported for patients developing radiation-induced cancers.
Methods
In our prospective study we determined the cumulative maximum entrance skin doses (MESD) and the incidence of epilation in 107 consecutive patients submitted to NEP between 2003 and 2007. We also extensively searched the literature and compared our results with the data we found.
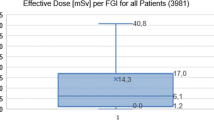
Results
The cumulative MESD due to NEP was above 3,000 mGy (range 3,101–5,421 mGy) in 18 patients. In 22 we observed partial epilation within 10 weeks from the initial NEP. Sixty cases of epilation after NEP have been previously reported in the literature. The average of the reported MESD was 4,241 mGy (range 2,000–6,640 mGy).
Conclusion
Physical dosimetry and the incidence of partial epilation indicate that about one fifth of the patients submitted to NEP received radiation doses comparable to those linked to the occurrence of tumors. The potential risks of developing tumors after a long delay, when compared to the immediate benefits of endovascular treatment of aneurysm and arteriovenous malformations (AVM) of the brain, do not counterindicate NEP, but increased awareness of the risk should help physicians and patients to make a fully informed decision when other treatments are available.



Similar content being viewed by others
References
Albert RE, Omran AR, Brauer EW, Dove DC, Cohen NC, Schmidt H, Baumring R, Morrill S, Schulz R, Baer RL (1966) Follow-up study of patients treated by x-ray for tinea capitis. Am J Public Health Nations Health 56:2114–2120
Alexander MD, Oliff MC, Olorunsola OG, Brus-Ramer M, Nickoloff EL, Meyers PM (2010) Patient radiation exposure during diagnostic and therapeutic interventional neuroradiology procedures. J Neuro Interv Surg 2:6–10. doi:10.1136/jnis.2009.000802
Avoidance of Radiation Injuries from Medical Interventional Procedures ICRP Publication 85. Ann ICRP 2000; 30
Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ, Bastir M, Rosas A, O’Higgins P (2010) Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology 254:326–341
Bastir M, Rosas A, O’Higgins P (2006) Craniofacial levels and the morphological maturation of the human skull. J Anat 209:637–654
Board of Radiation Effects Research Division on Earth and Life Sciences (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. National Academy Press, Washington, DC
Currie S, Mankad K, Goddard A (2011) Endovascular treatment of intracranial aneurysms: review of current practice. Postgrad Med J 87:41–50
D’Ercole L, Mantovani L, Thyrion FZ, Bocchiola M, Azzaretti A, Di Maria F, Saluzzo CM, Quaretti P, Rodolico G, Scagnelli P, Andreucci L (2007) A study on maximum skin dose in cerebral embolization procedures. AJNR Am J Neuroradiol 28:503–507
D’Ercole L, Thyrion FZ, Bocchiola M, Mantovani L, Klersy C (2010) Proposed local diagnostic reference levels in angiography and interventional neuroradiology and a preliminary analysis according to the complexity of the procedures. Phys Med. PMID:21074469
Diallo I, Haddy N, Adjadj E, Samand A, Quiniou E, Chavaudra J, Alziar I, Perret N, Guérin S, Lefkopoulos D, de Vathaire F (2009) Frequency distribution of second solid cancer locations in relation to the irradiated volume among 115 patients treated for childhood cancer. Int J Radiat Oncol Biol Phys 74:876–883
D’incan M, Roger H, Gabrillargues J, Mansard S, Parent S, Chazal J, Irthum B, Souteyrand P (2002) Radiation-induced temporary hair loss after endovascular embolization of the cerebral arteries: six cases. Ann Dermatol Venereol 129:703–706
Flint-Richter P, Sadetzki S (2007) Genetic predisposition for the development of radiation-associated meningioma: an epidemiological study. Lancet Oncol 8:403–410
Geleijns J, Wondergem J (2005) X-ray imaging and the skin: radiation biology, patient dosimetry and observed effects. Radiat Prot Dosimetry 114:121–125
Gkanatsios NA, Huda W, Peters KR (2002) Adult patient doses in interventional neuroradiology. Med Phys 29:717–723
Guglielmi G (1997) Endovascular treatment of aneurysms. History, development and application of current techniques. J Stroke Cerebrovasc Dis 6:246–248
Hayakawa M, Moritake T, Kataoka F, Takigawa T, Koguchi Y, Miyamoto Y, Akahane K, Matsumaru Y (2010) Direct measurement of patient’s entrance skin dose during neurointerventional procedure to avoid further radiation-induced skin injuries. Clin Neurol Neurosurg 112:530–536
http://www.ev3.net/neuro/us/embolic-coils. accessed on May 1, 2011
Huda W, Peters KR (1994) Radiation-induced temporary epilation after a neuroradiologically guided embolization procedure. Radiology 193:642–644
Imanishi Y, Fukui A, Niimi H, Itoh D, Nozaki K, Nakaji S, Ishizuka K, Tabata H, Furuya Y, Uzura M, Takahama H, Hashizume S, Arima S, Nakajima Y (2005) Radiation-induced temporary hair loss as a radiation damage only occurring in patients who had the combination of MDCT and DSA. Eur Radiol 15:41–46
Jung YH, Park SH, Kim YS, Hamm IS (2005) Six-year experience of endovascular embolization for intracranial aneurysms. J Korean Neurosurg Soc 38:190–195
Krasovec M, Trüeb RM (1998) Temporary roentgen epilation after embolization of a cerebral arteriovenous malformation. Hautarzt 49:307–309
Lee WS, Lee SW, Lee S, Lee JW (2004) Postoperative alopecia in five patients after treatment of aneurysm rupture with a Guglielmi detachable coil: pressure alopecia, radiation induced, or both? J Dermatol 31:848–851
Livingstone RS, Raghuram L, Korah IP, Raj DV (2003) Evaluation of radiation risk and work practices during cerebral interventions. J Radiol Prot 23:327–336
Longstreth WT Jr, Phillips LE, Drangsholt M, Koepsell TD, Custer BS, van Gehrels JA, Belle G (2004) Dental X-rays and the risk of intracranial meningioma: a population-based case-control study. Cancer 100:1026–1034
Maalej M, Frikha H, Kochbati L, Bouaouina N, Sellami D, Benna F, Gargouri W, Dhraief S, Nasr C, Daoud J, Hajji M, Fazaa B, Souissi R, Mokhtar I, Kamoun MR (2004) Radio-induced malignancies of the scalp about 98 patients with 150 lesions and literature review. Cancer Radiother 8:81–87
Marti N, Lopez V, Pereda C, Martin JM, Montesinos E, Jorda E (2008) Radiation-induced temporary alopecia after embolization of cerebral aneurysms. Dermatol Online J 14:19
Mistretta CA (2011) Sub-Nyquist acquisition and constrained reconstruction in time resolved angiography. Med Phys 38:2975–2985
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R (2002) International Subarachnoid Aneurysm Trial (ISAT) collaborative Group, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360:1267–1274
Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J, Collaborators ISAT (2009) Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 8:427–433
Momani MS, Shore-Freedman E, Collins BJ, Lubin J, Ron E, Schneider AB (2004) Familial concordance of thyroid and other head and neck tumors in an irradiated cohort: analysis of contributing factors. J Clin Endocrinol Metab 89:2185–2191
Mooney RB, McKinstry CS, Kamel HA (2000) Absorbed dose and deterministic effects to patients from interventional neuroradiology. Br J Radiol 73:745–751
Nannapaneni R, Behari S, Mendelow D, Gholkar A (2007) Temporary alopecia after subarachnoid haemorrhage. J Clin Neurosci 14:157–161
Neil S, Padgham C, Martin CJ (2010) A study of the relationship between peak skin dose and cumulative air kerma in interventional neuroradiology and cardiology. J Radiol Prot 30:659–672
Norbash AM, Busick D, Marks MP (1996) Techniques for reducing interventional neuroradiologic skin dose: tube position rotation and supplemental beam filtration. AJNR Am J Neuroradiol 17:41–49
Preston DL, Ron E, Yonehara S, Kobuke T, Fugii H, Kishikawa M, Tokunaga M, Tokuoka S, Mabuchi K (2002) tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst 94:1555–1563
Rinkel GJ, Algra A (2011) Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 10:349–356
Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I (2005) Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res 163:424–432
Sandborg M, Rossitti S, Pettersson H (2010) Local skin and eye lens equivalent doses in interventional neuroradiology. Eur Radiol 20:725–733
Saracci R, Samet J (2010) Commentary: Call me on my mobile phone…or better not?–a look at the INTERPHONE study results. Int J Epidemiol 39:695–698
Schneider AB, Ron E, Lubin J, Stovall M, Shore-Freedman E, Tolentino J, Collins BJ (2008) Acoustic neuromas following childhood radiation treatment for benign conditions of the head and neck. Neuro Oncol 10:73–78
Schueler BA, Kallmes DF, Cloft HJ (2005) 3D cerebral angiography: radiation dose comparison with digital subtraction angiography. AJNR Am J Neuroradiol 26:1898–1901
Shore RE, Moseson M, Harley N, Pasternack BS (2003) tumors and other diseases following childhood x-ray treatment for ringworm of the scalp (tinea capitis). Health Phys 85:404–408
Shvarts S, Sevo G, Tasic M, Shani M, Sadetzki S (2010) The tinea capitis campaign in Serbia in the 1950s. Lancet Infect Dis 10:571–576
Suzuki S, Furui S, Matsumaru Y, Nobuyuki S, Ebara M, Abe T, Itoh D (2008) Patient skin dose during neuroembolization by multiple-point measurement using a radiosensitive indicator. AJNR Am J Neuroradiol 29:1076–1081
Sznajder L, Abrahams C, Parry DM, Gierlowski TC, Shore-Freedman E, Schneider AB (1996) Multiple schwannomas and meningiomas associated with irradiation in childhood. Arch Intern Med 156:1873–1878
The 2007 Recommendations of the International Commission on Radiological Protection ICRP Publication 103 Ann ICRP 2007; 37
Thierry-Chef I, Simon SL, Land CE, Miller DL (2008) Radiation dose to the brain and subsequent risk of developing brain tumors in pediatric patients undergoing interventional neuroradiology procedures. Radiat Res 170:553–565
Thorat JD, Hwang PY (2007) Peculiar geometric alopecia and trigeminal nerve dysfunction in a patient after Guglielmi detachable coil embolization of a ruptured aneurysm. J Stroke Cerebrovasc Dis 16:40–42
Tosti A, Piraccini BM, Alagna G (1999) Temporary hair loss simulating alopecia areata after endovascular surgery of cerebral arteriovenous malformations: a report of 3 cases. Arch Dermatol 135:1555–1556
Vano E, Ubeda C, Martinez LC, Leyton F, Miranda P (2010) Paediatric interventional cardiology: flat detector versus image intensifier using a test object. Phys Med Biol 55:7287–7297
Wen CS, Lin SM, Chen Y, Chen JC, Wang YH, Tseng SH (2003) Radiation-induced temporary alopecia after embolization of cerebral arteriovenous malformations. Clin Neurol Neurosurg 105:215–217
Wiart J, Hadjem A, Gadi N, Bloch I, Wong MF, Pradier A, Lautru D, Hanna VF, Dale C (2005) Modeling of RF head exposure in children. Bioelectromag Suppl 7:S19–S30
Yonehara S, Brenner AV, Kishikawa M, Inskip PD, Preston DL, Ron E, Mabuchi K, Tokuoka S (2004) Clinical and epidemiologic characteristics of first primary tumors of the central nervous system and related organs among atomic bomb survivors in Hiroshima and Nagasaki, 1958–1995. Cancer 101:1644–1654
Zeeb H, Hammer GP, Langner I, Schafft T, Bennack S, Blettner M (2010) Cancer mortality among German aircrew: second follow-up. Radiat Environ Biophys 49:187–194
Acknowledgments
We thank Dr. Eloisa Arbustini, Dr. Gabriele Biella, Dr. Roberto Imberti, and Dr. Marco Marchionni for useful suggestions and corrections. We thank the participating patients and their families; this study would not have been possible without their cooperation.
Conflicts of interest
None.
Funding
This study was supported by Fondazione IRCCS Policlinico S. Matteo (Pavia, Italy) with funds awarded for intramural studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00701-012-1280-x.
Rights and permissions
About this article
Cite this article
Magrassi, L., Bongetta, D., D’Ercole, L. et al. Neuroembolization may expose patients to radiation doses previously linked to tumor induction. Acta Neurochir 154, 33–41 (2012). https://doi.org/10.1007/s00701-011-1209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1209-9