Abstract
Background
The spinal arteries are innervated by several systems that contribute to the control of spinal cord blood flow. The sensory fibers of upper cervical nerves have vasodilatatory effect on the anterior spinal arteries (ASA). Subarachnoid hemorrhage (SAH) causes severe vasospasm by various neurochemical mechanisms. We examined whether there is a relationship between the neuron density of the C3 dorsal root ganglion and the severity of ASA vasospasm in SAH.
Methods
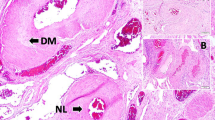
This study was conducted on 20 rabbits. Four of them were used as baseline group. Experimental SAH has been applied to all of 16 animals by injecting homologous blood into cisterna magna. After 20 days of injection, ASA and C3 dorsal root ganglia (C3DRG) were examined histopathologically. ASA volume values and normal and degenerated neuron densities of C3DRG were estimated stereologically and the results were analyzed statistically.
Results
The mean ASA volume was 1,010 ± 450 mm3, and the mean neuronal density of C3DRG was 10,500 ± 850 in all animals. The mean volume value of ASA was 970 ± 150 mm3, and the normal neuron density of C3DRG fell to 8,600 ± 400/mm3 in slight vasospasm group. In severe vasospasm-developed animals, mean volume value of ASA was 540 ± 90 mm3 and the normal neuron density of C3DRG fell to 5,500 ± 360/mm3. An inverse relationship between the degenerated neuronal density of the C3DRG and ASA volume values may indicate the severity of ASA vasospasm.
Conclusion
The neuron density of C3DRG may be an important factor on the regulation of ASA volume values and the continuation of spinal cord blood flow. Low neuron density of C3DRG may be considered as an important factor in the pathogenesis of severe ASA vasospasm in SAH.



Similar content being viewed by others
Abbreviations
- ASA:
-
Anterior spinal arteries
- DRG:
-
Dorsal root ganglia
- SAH:
-
Subarachnoid hemorrhage
References
Allen L, Foster RW, Morsan GP, Small RC (1986) The relaxant effect of nicorandil in guinea pig isolated trachealis. Br J Pharmacol 187:117–127
Arbab MAR, Wiklund L, Svendgaard NA (1986) Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience 19:695–708
Aydin MD, Dane S, Gundogdu C, Gursan N (2004) Neurodegenerative effects of monopolar electrocauterization on spinal ganglia in lumbar disc surgery. Acta Neurochir (Wien) 146:1125–1129
Aydin MD, Erdogan AR, Cevli SC, Gundogdu C, Dane S, Diyarbakirli S (2006) Ganglionary mechanisms of spasticity and ileus in cerebral hemorrhage: an experimental study. Int J Dev Neurosci 24:455–459
Aydin MD, Yildirim OS, Gundogdu C, Onder A, Okur A (2006) Thrombogenetic effect of facet denervation using in disc surgery on spinal radicular arteries: an experimental study. Minim Invasive Neurosurg 49(6):328–330
Bendsen E, Byskov AG, Laursen SB, Larsen H-PE, Andersen CY, Westergaard LG (2003) Number of germ cells and somatic cells in human fetal testes during the first weeks after sex differentiation. Hum Reprod 18:13–18
Buchanan KM, Elias LJ, Goplen GB (2000) Differing perspectives on outcome after subarachnoid hemorrhage: the patient, the relative, the neurosurgeon. Neurosurgery 46:831–838
Carter BS, Buckley D, Ferraro R, Rordorf G, Ogilvy CS (2000) Factors associated with reintegration to normal living after subarachnoid hemorrhage. Neurosurgery 46:1326–1333
Chang Z, Shen Z, Sun Y, Wang N, Cao D (1999) Early repair treatment of electrical burns and recovery of tendons and nerves. Report of 194 operations. Ann NY Acad Sci 888:327–333
Cruz-Orive LM, Weibel ER (1990) Recent stereological methods for cell biology: a brief survey. Am J Physiol 258:148–156
Edvinsson L (1975) Neurogenic mechanisms in the cerebrovascular bed: autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl 427:1–35
Groves MJ, Christopherson T, Giometto B, Scaravilli F (1997) Axotomyinduced apoptosis in adult rat primary sensory neurons. J Neurocytol 26:615–624
Gundersen HJ (1986) Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R Thompson. J Microsc 143:3–45
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Gundersen HJG (1977) Notes on the estimation of the numerical density of arbitrary particles: the edge effect. J Microsc 111:219–223
Hara H, Zhang QJ, Kuroyanagi T, Kobayashi S (1993) Parasympathetic cerebrovascular innervation: an anterograde tracing from the sphenopalatine ganglion in the rat. Neurosurgery 32:822–827
Hu P, McLachlan EM (2002) Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 112:23–38
Itoh T, Furukawa K, Kajiwara M, Kitamura K, Suzuki H, Ito Y, Kuriyama H (1981) Effects of 2-nicotinamidoethyl nitrate on smooth muscle cells and on adrenergic transmission in the guinea pig and porcine mesenteric arteries. J Pharmacol Exp Ther 218:260–270
Kassel NF, Sasaki T, Colohan A, Nazure G (1985) Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 16:562–572
Kassell NF, Drake CG (1982) Timing of aneurysm surgery. Neurosurgery 10:514–519
Kurth CD, Wagerle LC, Delivoria-Papadopoulos M (1988) Sympathetic regulation of cerebral blood flow during seizures in newborn lambs. Am J Physiol 255:563–568
Liu-Chen LY, Mayberg MR, Moskowitz MA (1983) Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res 268:162–166
Ma J, Novikov LN, Wiberg M, Kellerth JO (2001) Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp Brain Res 139:216–223
McKay HA, Brannstrom T, Wiberg M, Terenghi G (2002) Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res 142:308–318
Medlock MD, Dulebohn SC, Elwood PW (1992) Prophylactic hypervolemia without calcium channel blockers in early aneurysm surgery. Neurosurgery 30:12–16
Oliveira AL, Risling M, Negro A, Langone F, Cullheim S (2002) Apoptosis of spinal interneurons induced by sciatic nerve axotomy in the neonatal rat is counteracted by nerve growth factor and ciliary neurotrophic factor. J Comp Neurol 447:381–933
Origitano TC, Wascher TM, Reichman OH, Anderson DE (1990) Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution (“triple-H” therapy) after subarachnoid hemorrhage. Neurosurgery 27:729–739
Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T (2001) Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6 J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience 105:249–263
Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136
Taiushev KG (1989) Segment-by-segment histological analysis of the cervical part of the spinal cord, roots of the cerebrospinal nerves and ganglia in severe cranio-cerebral trauma. Arkh Anat Gistol Émbriol 96:16–23
Tuor UI (1990) Local distribution of the effects of sympathetic stimulation on cerebral blood flow in the rat. Brain Res 529:224–231
Waters A, Harder DR (1985) Altered membrane properties of cerebral vascular smooth muscle following subarachnoid hemorrhage: an electrophysiological study. I. Changes in resting membrane potential (Em) and effect on the electrogenic pump potential contribution to Em. Stroke 16: 990–997
Yasargil MG (1999) A legacy of microneurosurgery: memoirs, lessons, and axioms. Neurosurgery 45(5):1025–1092
Yasargil MG (1997) The advent of microsurgery. Mt Sinai J Med 64(3):164–165
Acknowledgment
This study was presented as a poster presentation at Turkish Neurosurgical Society, 24th Annual Meeting in Antalya, Turkey.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This is a novel study of the effect of vasospasm in subarachnoid hemorrhage in an experimental rabbit model. The authors carefully designed a study that would evaluate the relationship of neuron density in the C3 DRG and the severity of anterior spinal artery (ASA) vasospasm. This was performed by direct injection of homologous blood into the cisterna magna, followed by nerve sectioning and histopathology. Twenty animals in total were studied, four of which acted as controls. After 20 days, the animals were sacrificed and histopathology was performed using the physical dissector method for neuron density and a microtome was used for measurement of the ASA. This group attempts to show that an inverse relationship exists between neuronal density of the C3-DRG and ASA volume. We read this study with great interest, and as there is current interest in cervical spinal cord stimulation in SAH, this type of data may prove to be helpful in the clarification of the mechanisms.
1. Yoshihiro TAKANASHI and Masamichi SHINONAGA; “Spinal Cord Stimulation for Cerebral Vasospasm as Prophylaxis”, Neurologia medico-chirurgica, Vol. 40, pp.352–357 (2000).
Christopher Loftus
Philadelphia. USA
An erratum to this article is available at http://dx.doi.org/10.1007/s00701-010-0844-x.
Rights and permissions
About this article
Cite this article
Kanat, A., Yilmaz, A., Aydin, M.D. et al. Role of degenerated neuron density of dorsal root ganglion on anterior spinal artery vasospasm in subarachnoid hemorrhage: experimental study. Acta Neurochir 152, 2167–2172 (2010). https://doi.org/10.1007/s00701-010-0793-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0793-4