Abstract
Purpose
Carmustine (1,3-bis[2-chloroetyl]-1-nitrosurea (BCNU)) wafers are approved for the local treatment of newly diagnosed and recurrent malignant glioma. Reassuring data on both safety and efficacy of treatment have been previously reported by phase III studies. Although most of related adverse events are reported in the first few months after surgery, there is a lack in the literature of radiological data regarding this period. Few anecdotal experiences have been reported about surgical bed cyst occurrence. The aim of our study is to analyse the radiological course of patients treated with wafers implantation focusing on the relationship between radiological data, and in particular bed cyst occurrence, and safety data.
Methods
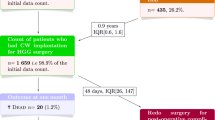
Forty-three patients affected by malignant glioma underwent surgical removal and BCNU wafers implantation at the Department of Neurosurgery of Padova from April 2007 to October 2009. Safety data were collected according to previously reported phase III studies. Patients underwent clinical and radiological evaluation (MRI) postoperatively, then before discharge, at 1 month, then every 2 months. In the study were included only patients whose both 1- and 3-month MRIs were available. Finally, 36 out of 43 patients were available for the revision.
Findings
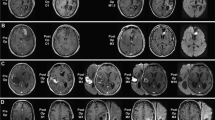
Fifty-eight percent of patients treated with BCNU wafers presented a bed cyst of the surgical cave at the 1-month MRI. Forty-eight percent of them were symptomatic. Conversely, among patients who presented one or more adverse event (27%), bed cyst was detected in up to 90% of cases (OR 7.35), being intracranial hypertension more frequently associated (OR 7.35; p value <0.05). In general, cysts presented a benign behaviour in the sense that patients promptly improved with corticosteroid treatment, never required surgery, never reported permanent neurological deficits.
Conclusions
Surgical bed cyst occurrence in BCNU wafer-treated patients resulted more frequent than expected. Familiarity with the event is important to correctly handle a possible evolving phenomenon. However, only further larger experiences and prospective studies could reveal how the understanding of such event might be helpful to improve safety data.



Similar content being viewed by others
References
Asher AL (2007) Prospective analysis of temozolomide as adjuvant to Gliadel and radiation in newly diagnosed malignant glioma. Abstract presented at the Annual Meeting of the American Association of Neurological Surgeons, Washington, DC, 2007
Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H (2008) Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol 15:2887–2893
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Clifford Schold S (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 345:1008–1012
Dal Pan G, Butler L, Schactman M (1997) Preliminary analysis of the GLIADEL treatment protocol. Abstract presented at the ASCO Annual Meeting, Atlanta, GA, 1997
Darakchiev BJ, Albright RE, Breneman JC, Warnick RE (2008) Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg 108:236–242
Eisai Inc. Gliadel Wafer: Heath Care Professionals. Available at: http://www.gliadel.com/docs/pdf/Gliadel_PI.pdf [Last accessed 2 February 2010]
Engelhard HH (2000) Tumor bed cyst formation after BCNU wafer implantation: report of two cases. Surg Neurol 53:220–224
Engelhard HH, Maher de Leon ME, Rozental JM (1998) Tumor bed cyst formation after BCNU wafer implantation. Abstract presented at the Annual Meeting of the Congress of Neurological Surgeons, Seattle, WA, 1998
Giese A, Bock HC, Kantelhardt SR, Rohde V (2010) Risk management in the treatment of malignant gliomas with BCNU wafer implants. Cent Eur Neurosurg Jan 8 doi:10.1055/s-0029-1242775
Gonza´lez Vidal D (2006) The safety of Gliadel implants during the indication period in recurrent surgery in the treatment of glioblastoma multiforme [abstract]. Neurocirugıa 17:77–78
Gururangan S, Cokgor L, Rich JN, Edwards S, Affronti ML, Quinn JA, Herndon JE 2nd, Provenzale JM, McLendon RE, Tourt-Uhlig S, Sampson JH, Stafford-Fox V, Zaknoen S, Early M, Friedman AH, Friedman HS (2001) Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro-Oncology 3:246–250
Kleinberg LR, Weingart J, Burger P, Grossman CK, SA Li K, Olivi A, Wharam MD, Brem H (2004) Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest 22:1–9
LaRocca R, Glisson S, Hargis J, Petruska D, Villanueva W, Morassutti D, Horne D, Amin-Zimmerman F (2005) High-grade glioma treated with surgery; carmustine wafer; postoperative radiation; and procarbazine, lomustine, and vincristine chemotherapy. Neurosurg Q 15:167–171
McGirt MJ, Villavicencio AT, Bulsara KR, Friedman HS, Friedman AH (2002) Management of tumor bed cysts after chemotherapeutic wafer implantation. report of four cases. J Neurosurg 96:941–945
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quiñones-Hinojosa A (2009) Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110(3):583–588
McGovern PC, Lautenbach E, Brennan PJ, Lustig RA, Fishman NO (2003) Risk factors for post-craniotomy surgical site infection after 1, 3-bis(2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis 36:759–765
NCCN (2009) NCCN guidelines. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [Last accessed 2 February 2010]
Pan E, Mitchell SB, Tsai JS (2008) A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neuro-oncol 88:353–357
Sabel M, Giese A (2008) Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin. 2008 Oct 20. doi:10.1185/03007990802508180
Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M, Schiff D (1999) Morbidity and survival after 1, 3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case matched cohort series. Neurosurgery 45:17–22
Tait MJ, Critchley GR (2006) Experience of revision craniotomy for debulking of high grade gliomas in 27 patients in a single UK centre, with and without insertion of carmustine wafers. Meeting of the Society of British Neurosurgeons, Preston, UK, 2006
Uff CEG, Bradford R (2005) Use of Gliadel (BCNU) Wafers in high grade glioma. Abstract presented at the meeting of the Society of British Neurosurgeons, Plymouth, UK, 2005
Volc D, Jellinger K, Flament H, Böck F, Klumair J (1981) Cerebral space-occupying cysts following radiation and chemotherapy of malignant gliomas. Acta Neurochir (Wien) 57:177–193
Weingart J, Grossman SA, Carson KA, Fisher JD, Delaney SM, Rosenblum ML, Olivi A, Judy K, Tatter SB, Dolan ME (2007) Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol 25:399–404
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z (2003) A Phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 5:79–88
Acknowledgments
This work was supported in part by Grant Ricerca Sanitaria Finalizzata 285/08 of Regione Veneto.
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Della Puppa, A., Rossetto, M., Ciccarino, P. et al. The first 3 months after BCNU wafers implantation in high-grade glioma patients: clinical and radiological considerations on a clinical series. Acta Neurochir 152, 1923–1931 (2010). https://doi.org/10.1007/s00701-010-0759-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0759-6