Abstract
Object
5-aminolevulinic acid (5-ALA) has gained importance as an intraoperative photodynamic diagnostic agent for the extirpation of malignant gliomas. The application of this technique for resection of meningiomas has barely been explored. The aim of this study was to evaluate the utility of 5-ALA-induced fluorescence as a visual tool in meningioma resection and its correlation with histological findings.
Methods
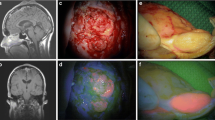
A total of 33 consecutive patients undergoing resection of intracranial meningiomas from December 2007 to August 2009 were included in this study. After confirmation of normal liver function, 5-ALA was administered orally (20 mg/kg) within 3–5 h prior to skin incision. All cases were operated on using standard microsurgical and neuronavigation-guided techniques. Intraoperative 440 nm fluorescence was applied periodically during and at the end of resection in order to detect tumor-infiltrated sites. The fluorescence of the tumor was evaluated intraoperatively by the surgeon and confirmed by subsequent video analysis.
Results
A total of 32 (97%) patients presented with benign meningiomas (WHO I–II). In 1 (3%) patient, histological anaplastic signs (WHO III) could be demonstrated. 5-ALA-induced fluorescence of the tumor was confirmed in a total of 31 (94%) patients. The fluorescence did not correlate with the histological findings (n = 30 WHO I–II, n = 1 WHO grade III) or with preoperative brain edema and administration of steroids. A total resection could be postoperatively demonstrated in 25 (76%) patients. No adverse effects attributable to 5-ALA occurred.
Conclusions
5-ALA-induced fluorescence is a useful and promising intraoperative tool for the visualization of meningioma tissue. The novel findings demonstrated in this study in terms of high fluorescence and poor correlation with histological findings highlight the usefulness of this technique as a routine visual tool to achieve optimal resection of meningiomas.



Similar content being viewed by others
References
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT Jr, Barker FG 2nd (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60, discussion 60
Ayerbe J, Lobato RD, de la Cruz J, Alday R, Rivas JJ, Gomez PA, Cabrera A (1999) Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien) 141:921–932
Bitzer M, Nagele T, Geist-Barth B, Klose U, Gronewaller E, Morgalla M, Heiss E, Voigt K (2000) Role of hydrodynamic processes in the pathogenesis of peritumoral brain edema in meningiomas. J Neurosurg 93:594–604
Bondy M, Ligon BL (1996) Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 29:197–205
Brown M, Schrot R, Bauer K, Letendre D (2009) Incidence of first primary central nervous system tumors in California, 2001–2005. J Neurooncol 94(2):249–261
Chan RC, Thompson GB (1984) Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg 60:52–60
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57:1088–1095, discussion 1088–1095
Colombo F, Casentini L, Cavedon C, Scalchi P, Cora S, Francescon P (2009) Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery 64:A7–A13
Duffner F, Ritz R, Freudenstein D, Weller M, Dietz K, Wessels J (2005) Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol 71:107–111
El-Sharabasy MM, El-Waseef AM, Hafez MM, Salim SA (1992) Porphyrin metabolism in some malignant diseases. Br J Cancer 65:409–412
Ennis SR, Novotny A, Xiang J, Shakui P, Masada T, Stummer W, Smith DE, Keep RF (2003) Transport of 5-aminolevulinic acid between blood and brain. Brain Res 959:226–234
Fotinos N, Campo MA, Popowycz F, Gurny R, Lange N (2006) 5-Aminolevulinic acid derivatives in photomedicine: characteristics, application and perspectives. Photochem Photobiol 82:994–1015
Gabeau-Lacet D, Aghi M, Betensky RA, Barker FG, Loeffler JS, Louis DN (2009) Bone involvement predicts poor outcome in atypical meningioma. J Neurosurg 111(3):464–471
Hebeda KM, Saarnak AE, Olivo M, Sterenborg HJ, Wolbers JG (1998) 5-Aminolevulinic acid induced endogenous porphyrin fluorescence in 9L and C6 brain tumours and in the normal rat brain. Acta Neurochir (Wien) 140:503–512, discussion 512–503
Hoffman S, Propp JM, McCarthy BJ (2006) Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol 8:27–37
Iinuma S, Farshi SS, Ortel B, Hasan T (1994) A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer 70:21–28
Ildan F, Erman T, Gocer AI, Tuna M, Bagdatoglu H, Cetinalp E, Burgut R (2007) Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base 17:157–171
Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M (1992) Peritumoral brain edema in meningiomas—influence of vascular supply on its development. Neurosurgery 31:179–185
Jaaskelainen J (1986) Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 26:461–469
Jaaskelainen J, Haltia M, Servo A (1986) Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol 25:233–242
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Johnson WD, Loredo LN, Slater JD (2008) Surgery and radiotherapy: complementary tools in the management of benign intracranial tumors. Neurosurg Focus 24:E2
Kajimoto Y, Kuroiwa T, Miyatake S, Ichioka T, Miyashita M, Tanaka H, Tsuji M (2007) Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J Neurosurg 106:1070–1074
Kantelhardt SR, Diddens H, Leppert J, Rohde V, Huttmann G, Giese A (2008) Multiphoton excitation fluorescence microscopy of 5-aminolevulinic acid induced fluorescence in experimental gliomas. Lasers Surg Med 40:273–281
Kennedy JC, Pottier RH (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B 14:275–292
Kollova A, Liscak R, Novotny J Jr, Vladyka V, Simonova G, Janouskova L (2007) Gamma knife surgery for benign meningioma. J Neurosurg 107:325–336
Krammer B, Plaetzer K (2008) ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 7:283–289
Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T (2005) Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery 56:546–559, discussion 546–559
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Madsen C, Schroder HD (1997) Ki-67 immunoreactivity in meningiomas—determination of the proliferative potential of meningiomas using the monoclonal antibody Ki-67. Clin Neuropathol 16:137–142
Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I (2008) Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery 62:102–103, discussion 103–104
Morokoff AP, Zauberman J, Black PM (2008) Surgery for convexity meningiomas. Neurosurgery 63:427–433, discussion 433–424
Mustajoki P, Timonen K, Gorchein A, Seppalainen AM, Matikainen E, Tenhunen R (1992) Sustained high plasma 5-aminolaevulinic acid concentration in a volunteer: no porphyric symptoms. Eur J Clin Investig 22:407–411
Nakano T, Asano K, Miura H, Itoh S, Suzuki S (2002) Meningiomas with brain edema: radiological characteristics on MRI and review of the literature. Clin Imaging 26:243–249
Nakasu S, Fukami T, Jito J, Nozaki K (2009) Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol 26:69–72
Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF (2000) Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem 75:321–328
Olivo M, Wilson BC (2004) Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int J Oncol 25:37–45
Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM (1997) 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol 65:235–251
Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, Nesland JM (1997) 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 79:2282–2308
Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM (1997) Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21:1455–1465
Simis A, de Aguiar PH Pires, Leite CC, Santana PA Jr, Rosemberg S, Teixeira MJ (2008) Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol 70:471–477, discussion 477
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Stafford SL, Perry A, Suman VJ, Meyer FB, Scheithauer BW, Lohse CM, Shaw EG (1998) Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 73:936–942
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ (1998) Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien) 140:995–1000
Stummer W, Stocker S, Novotny A, Heimann A, Sauer O, Kempski O, Plesnila N, Wietzorrek J, Reulen HJ (1998) In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B 45:160–169
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–525, discussion 525–516
Terr L, Weiner LP (1983) An autoradiographic study of delta-aminolevulinic acid uptake by mouse brain. Exp Neurol 79:564–568
Tsai JC, Hsiao YY, Teng LJ, Chen CT, Kao MC (1999) Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg Med 24:296–305
Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y, Kondo K, Miyajima Y, Fujii K (2007) Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo) 47:210–213, discussion 213–214
Webber J, Kessel D, Fromm D (1997) Plasma levels of protoporphyrin IX in humans after oral administration of 5-aminolevulinic acid. J Photochem Photobiol B 37:151–153
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors gave 5-ALA (Gliolan) 3–5 h preoperatively to 33 patients with intracranial meningiomas and observed fluorescence in 31/33—with no correlation to various clinical characteristics. This is an important pilot study that should be verified by other neurosurgical teams. Why meningioma tissue becomes fluorescent after 5-ALA requires research at tissue/cellular/molecular level. Further studies—if 5-ALA becomes cheaper—will determine whether and when this approach improves the degree of removal of meningiomas. Furthermore, 5-ALA should be tested in many other benign tumors of the CNS, including schwannomas, adenomas, and some 20 different types of grade I gliomas such as pilocytic astrocytomas.
Most importantly, 5-ALA painting is just a beginning to the new era of advanced optics in neurosurgery—clinical testing and verification of various targeted painting molecules in different instances.
Juha E Jääskeläinen
Neurosurgery/NeuroCenter/Kuopio University Hospital
Kuopio Finland
Rights and permissions
About this article
Cite this article
Coluccia, D., Fandino, J., Fujioka, M. et al. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir 152, 1711–1719 (2010). https://doi.org/10.1007/s00701-010-0708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0708-4