Abstract
Purpose
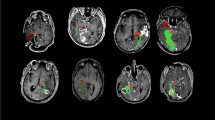
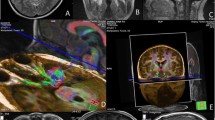
Diffusion tensor tractography provides useful information regarding the surgical strategy for brain tumors. The goal of the present study was to analyze relationships between visual field deficits and the locations of brain tumors compared with optic tracts as visualized by tractography, and compared with the calcarine fissure.
Methods
Subjects comprised 11 patients with brain tumor in the occipital lobe or atrium of the lateral ventricle who underwent surgery between October 2006 and February 2009. Tumors were categorized as Type A, with almost all the optic tract in the occipital lobe or atrium of the lateral ventricle running close to and stretched by the brain tumor; and Type B, with the optic tract running at least partially distant to the brain tumor and remaining unstretched.
Results
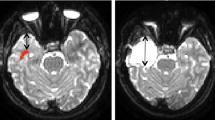
Those type A optic tracts that were laterally compressed by brain tumors (Cases 1–3) displayed hemianopsia after surgery. When the brain tumor was located rostro-medial to the calcarine fissure and optic tracts were compressed caudally by the tumor, lower quadrant hemianopsia remained after surgery (Cases 4, 5). In other cases, the visual field remained or improved to normal after surgery.
Conclusion
The relationship between optic tracts or the calcarine fissure, and brain tumors in the occipital lobe or atrium of the lateral ventricle is related to visual field deficits after surgery. In particular, those Type A optic tracts that are compressed laterally show hemianopsia of the visual field after surgery.


Similar content being viewed by others
References
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757
Berman JI, Berger MS, Chung SW, Nagarajan SS, Henry RG (2007) Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg 107:488–494
Burgel U, Schormann T, Schleicher A, Zilles K (1999) Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. Neuroimage 10:489–499
Catani M, Jones DK, Donato R, Ffytche DH (2003) Occipito-temporal connections in the human brain. Brain 126:2093–2107
Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimoi JS, McKinstry RC, Burton H, Raichle ME (1999) Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 96:10422–10427
DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J (1996) Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA 93:2382–2386
Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA (2003) Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3:586–598
Engel SA, Glover GH, Wandell BA (1997) Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex 7:181–192
Goebell E, Paustenbach S, Vaeterlein O, Ding XQ, Heese O, Fiehler J, Kucinski T, Hagel C, Westphal M, Zeumer H (2006) Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology 239:217–222
Jones EG (1999) Making brain connections: neuroanatomy and the work of TPS Powell, 1923–1996. Annu Rev Neurosci 22:49–103
Lee JS, Han MK, Kim SH, Kwon OK, Kim JH (2005) Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. Neuroimage 26:771–776
Malis LI (2001) Nuances in acoustic neuroma surgery. Neurosurgery 49:337–341
Mori S, Frederiksen K, van Zijl PCM, Stieltjes B, Kraut MA (2002) Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol 51:377–380
Nguyen TH, Yoshida M, Stievenart JL, Iba-Zizen MT, Bellinger L, Abanou A, Kitahara K, Cabanis EA (2005) MR tractography with diffusion tensor imaging in clinical routine. Neuroradiology 47:334–343
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fiber tract navigation. Neurosurgery 58:292–303
Pagani E, Filippi M, Rocca MA, Horsfield MA (2005) A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 26:258–265
Pertier J, Travers N, Destrieux C, Velut S (2006) Optic radiations: a microsurgical anatomical study. J Neurosurg 105:294–300
Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003) Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49:177–182
Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell BH (1995) Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–893
Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K (2005) Fibers connecting the primary motor and sensory area play a role in grasp stability of the hand. Neuroimage 25:936–941
Shinoura N, Yamada R, Kodama T, Suzuki Y, Takahashi M, Yagi K (2005) Preoperative fMRI, tractography and continuous task during awake surgery for maintenance of motor function following surgical resection of metastatic tumor spread to the primary motor area. Minim Invasive Neurosurg 48:85–90
Shinoura N, Yoshida M, Yamada R, Tabei Y, Saito K, Suzuki Y, Takayama Y, Yagi K (2009) Awake surgery with continuous task for resection of brain tumors in the primary motor area. J Clin Neurosci 16:188–194
Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H (2007) Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. Neuroimage 34:985–995
Yu CS, Li KC, Xuan Y, Ji XM, Qin W (2005) Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol 56:197–204
Acknowledgments
This work was supported by the Foundation for Tokyo Metropolitan Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinoura, N., Suzuki, Y., Yamada, R. et al. Relationships between brain tumor and optic tract or calcarine fissure are involved in visual field deficits after surgery for brain tumor. Acta Neurochir 152, 637–642 (2010). https://doi.org/10.1007/s00701-009-0582-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0582-0