Abstract
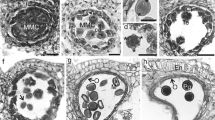
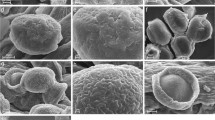
The genus Adansonia (baobabs, Malvaceae) includes nine tropical species grouped in the Brevitubae, Longitubae and Adansonia sections. The ultrastructure of pollen from eight species of baobabs was studied using transmission electron microscopy. The pollen grains correspond to 3-colporate and 4-colporate types. The ectexine is formed by a perforate tectum with isolated spinules. An important distinguishing feature between the Brevitubae section and the Longitubae and Adansonia sections was observed in the infratectum. The apertural region was similar in all the species studied here. However, the most unusual structure was observed in the apertural margins where the ectexine consisted of a thick lamellate annulus and the endexine had a cracked appearance; the intine was composed of a well-developed oncus under the endopore with unusual fibrillar structures and the outer layer had a remarkable structure consisting of columns. Despite the few differences observed in the structure of the pollen wall within the genus, an original arrangement of the structure found in the aperture of the Adansonia pollen grains studied provides additional information about the new types of apertural structures. This type of sporoderm adds to our knowledge of the diversity of angiosperm pollen. Moreover, this apertural structure is probably an adaptation that occurs during the formation of the pollen tube and of harmomegathy.










Similar content being viewed by others
References
Alverson W, Karol K, Baum D et al (1998) Circumscription of the Malvales and relationships to other Rosidae: evidence from rbcL sequence data. Am J Bot 85:876
Alverson WS, Whitlock BA, Nyffeler R et al (1999) Phylogeny of the core Malvales: evidence from ndhF sequence data. Am J Bot 86:1474–1486
Andriafidison D, Andrianaivoarivelo RA, Ramilijaona OR et al (2005) Nectarivory by endemic malagasy fruit bats during the dry season. Biotropica 38:85–90
Baum DA (1995a) The comparative pollination and floral biology of baobabs (Adansonia-Bombacaceae). Ann Mo Bot Gard 82:322–348
Baum DA (1995b) A systematic revision of Adansonia (Bombacaceae). Ann Mo Bot Gard 82:440–470
Baum DA, Small RL, Wendel JF (1998) Biogeography and floral evolution of baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. Syst Biol 47:181–207
Baum DA, Smith SD, Yen A et al (2004) Phylogenetic relationships of Malvatheca (Bombacoideae and Malvoideae; Malvaceae sensu lato) as inferred from plastid DNA sequences. Am J Bot 91:1863–1871
Blackmore S, van Helvoort HAM, Punt W (1984) On the terminology, origins and functions of caveate pollen in Compositae. Rev Palaeobot Palynol 43:293–301
Edlund AF (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:S84–S97
Erdtman G (1952) Pollen morphology and plant taxonomy angiosperms. Almqvist and Wiksell, Stockholm
Fuchs HP (1967) Pollen morphology of the family Bombacaceae. Rev Palaeobot Palynol 3:119–132
Furness CA, Rudall PJ (2000) Aperture absence in pollen of monocotyledons. In: Harley MM, Morton CM, Blackmore S (eds) Pollen Spores Morphol. Biol. Royal Botanic Gardens, Kew, pp 249–257
Furness CA, Rudall PJ (2001) Pollen and anther characters in monocot systematics. Grana 40:17–25
Goldblatt P, Thomas AL, Suárez-Cervera M (2004) Phylogeny of the Afro-Madagascan Aristea (Iridaceae) revisited in the light of new data on pollen morphology. Bot J Linn Soc 144:41–68
Heslop-Harrison Y (1977) The pollen-stigma interaction: pollen-tube penetration in Crocus. Ann Bot 41:913–922
Hesse M (2000) Pollen wall stratification and pollination. Plant Syst Evol 222:1–17
Jaeger P (1945) Epanouissement et pollinisation de la fleur du baobab. Comptes Rendus Hebd Séances Académie Sci 220:369–371
Leong Pock Tsy J-M, Lumaret R, Mayne D et al (2009) Chloroplast DNA phylogeography suggests a West African centre of origin for the baobab, Adansonia digitata L. (Bombacoideae, Malvaceae). Mol Ecol 18:1707–1715
Leong Pock Tsy J-M, Lumaret R, Flaven-Noguier E et al (2013) Nuclear microsatellite variation in Malagasy baobabs (Adansonia, Bombacoideae, Malvaceae) reveals past hybridization and introgression. Ann Bot 112:1759–1773
Marquez J, Seoane-Camba JA, Suarez-Cervera M (1997) The role of the intine and cytoplasm in the activation and germination processes of Poaceae pollen grains. Grana 36:328–342
Nilsson S, Robyns A (1986) Bombacaceae Kunth. World Pollen Spore Flora 14:1–59
Osborn JM, Taylor TN, Schneider EL (1991) Pollen morphology and ultrastructure of Cabombaceae: correlations with pollination biology. Am J Bot 78:1367–1378
Owen J (1974) A contribution to the ecology of the African baobab (Adansonia digitata L.). Savanna 3:1–12
Pacini E, Hesse M (2005) Pollenkitt—its composition, forms and functions. Flora 200:399–415
Payne WW (1981) Structure and function in angiosperm pollen wall evolution. Rev Palaeobot Palynol 35:39–59
Pettigrew FRS, Jack D, Bell KL et al (2012) Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Malvaceae: Bombacoideae). Taxon 61:1240–1250
Presting D, Straka H, Friedrich B (1983) Palynologia Madagassica et Mascarenica. Familien 128 bis 146. Trop Subtrop Pflanzenwelt 44:1–93
Ryckewaert P, Razanamaro O, Rasoamanana E et al (2011) Les Sphingidae, probables pollinisateurs des baobabs malgaches. Bois For Trop 307:57–68
Start AN (1972) Pollination of the baobab (Adansonia digitata L.) by the fruit bat Rousettus aegiptiacus E. Geoffroy. East Afr Wildl J 10:71–72
Stroo A (2000) Pollen morphological evolution in bat pollinated plants. Plant Syst Evol 222:225–242
Suárez-Cervera M, Le Thomas A, Goldblatt P, et al. (2000) The channelled intine of Aristea major: ultrastructural modifications during development, activation and germination. In: Harley MM, Morton CM, Blackmore S (eds) Pollen Spores Morphol. Biol. Royal Botanic Gardens, Kew, pp 57–71
Suárez-Cervera M, Gillespie L, Arcalís E et al (2001) Taxonomic significance of sporoderm structure in pollen of Euphorbiaceae: tribes Plukenetieae and Euphorbieae. Grana 40:78–104
Suárez-Cervera M, Takahashi Y, Vega-Maray A, Seoane-Camba JA (2003) Immunocytochemical localization of Cry j 1, the major allergen of Cryptomeria japonica (Taxodiaceae) in Cupressus arizonica and Cupressus sempervirens (Cupressaceae) pollen grains. Sex Plant Reprod 16:9–15
Tanaka N, Uehara K, Murata J (2004) Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. J Plant Res 117:265–276
Taylor TN, Levin DA (1975) Pollen morphology of Polemoniaceae in relation to systematic and pollination systems: scanning electronic microscopy. Grana 15:91–112
Thiéry JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6:987–1018
Ulrich S, Hesse M, Bröderbauer D et al (2012) Schismatoglottis and Apoballis (Araceae: Schismatoglottideae): a new example for the significance of pollen morphology in Araceae systematics. Taxon 61:281–292
Vaishampayan N, Sharma YN (1981) On the pollen morphology of the genus Adansonia Linn. Curr Sci 50:919
Vega-Maray AM, FernáNdez-GonzáLez D, Valencia-Barrera R et al (2003) Ultrastructural modifications in the apertural intine of Parietaria judaica L. (Urticaceae) pollen during the early stages of hydration. Grana 42:220–226
Volkova OA, Severova EE, Polevova SV (2013) Structural basis of harmomegathy: evidence from Boraginaceae pollen. Plant Syst Evol 299:1769–1779
von Balthazar M, Schönenberger J, Alverson WS et al (2006) Structure and evolution of the androecium in the Malvatheca clade (Malvaceae s.l.) and implications for Malvaceae and Malvales. Plant Syst Evol 260:171–197
Weber M, Ulrich S (2010) The endexine: a frequently overlooked pollen wall layer and a simple method for detection. Grana 49:83–90
Wickens GE (2008) The baobabs: pachycauls of Africa, Madagascar and Australia. Springer, Berlin
Xu F-X, Kirchoff BK (2008) Pollen morphology and ultrastructure of selected species of Magnoliaceae. Rev Palaeobot Palynol 150:140–153
Acknowledgments
The authors are grateful to Pat Lowe for the collection of pollen sample of A. gregorii in Australia, to the Centre de Ressources en Imagerie Cellulaire (CRIC) Montpellier assistance with the SEM observation and to the Parc Cíentific de Barcelona (PCB) for preparation of the material for TEM. We also thank Karen Newby and Daphne Goodfellow for their careful revision of our English, and Cecile Fovet-Rabot for her support. This work received financial support from the International Foundation for Sciences (IFS) and Fondation pour la Recherche sur la Biodivesrité (FRB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasoamanana, E.N., Razanamaro, O., Ramavovololona, P. et al. Pollen wall ultrastructure of the genus Adansonia L. species. Plant Syst Evol 301, 541–554 (2015). https://doi.org/10.1007/s00606-014-1091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1091-z