Abstract
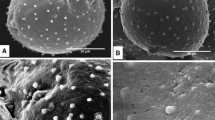
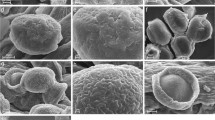
Upon release from the anther, pollen grains can be exposed to dry environment and dehydrate. To survive in dry conditions, the pollen wall possesses the ability to fold itself due to water loss-harmomegathic mechanism. Apertures seem to function as the primary elements of harmomegathy as they are more elastic than the remainder of the pollen wall. Contribution of other sporoderm structures, surface features, and pseudocolpi in harmomegathy are usually not considered in palynological studies. The nature of pseudocolpi has not been properly understood until now, partly because of common use of acetolysis method as a standard procedure. Different structures involved in the harmomegathy mechanism were studied in Cryptantha celosioides, Cryptantha coryi, Heliotropium europaeum, Myosotis palustris, Rindera bungei, and Rindera tetraspis. Scanning electron microscopy was used to study harmomegathy in hydrated and dehydrated pollen grains. In addition, transmission electron microscopy was used to elucidate the ultrastructural basis of pseudocolpi and other harmomegathic structures with special attention to intine structure. Our data reveal that additional flexibility of the pollen wall in Boraginaceae is provided by pseudocolpi, rugulate surface, tectate–columellate ultrastructure, and a transverse groove. Curious triangular polar poroid areas are described in M. palustris.





Similar content being viewed by others
References
Bigazzi M, Selvi F (1998) Pollen morphology in the Boragineae (Boraginaceae) in relation to the taxonomy of the tribe. Pl Syst Evol 213:121–151
Blackmore S, Barnes SH (1986) Harmomegathis mechanisms in pollen grains. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 137–149
Booi M, Punt W, Hoen PP (2003) The northern European pollen flora, 68. Lythraceae. Rev Palaebot Palynol 123:163–180
Corner EJH (1958) Transference of function. J Linn Soc (Zool) 44:33–40
Erdtman G (1960) The acetolysis method. A revised description. Sven Bot Tidskr 53:561–562
Erdtman G (1966) Pollen morphology and plant taxonomy: angiosperms. Hafner Publishing Co., New York, p 553
Halbritter H, Hesse M (2004) Principal modes of infoldings in tricolp(or)ate angiosperm pollen. Grana 43:1–14
Hargrove L, Simpson MG (2003) Ultrastructure of heterocolpate pollen in Cryptantha (Boraginaceae). Int J Plant Sci 164(1):137–151
Heslop-Harrison J (1979a) Pollen walls as adaptive systems. Ann Missouri Bot Gard 66:813–829
Heslop-Harrison J (1979b) An interpretation of the hydrodynamics of pollen. Amer J Bot 66(6):737–743
Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107:1–78
Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S (2009) Pollen terminology: an illustrated handbook. Springer, Wien, p 266
Katifori E, Albien S, Cerda E, Nelson DR, Dumais J (2010) Foldable structures and the natural design of pollen grains. PNAS 107(17):7635–7639
Muller J (1979) Form and function in angiosperm pollen. Ann Missouri Bot Gard 66:593–632
Muller J (1981) Exine architecture and function in some Lythraceae and Sonneratiaceae. Rev Palaebot Palynol 35:93–123
Nowicke JW, Skvarla JJ (1974) A palynological investigation of the genus Tournefortia (Boraginaceae). Amer J Bot 61(9):1021–1036
Patel VC, Skvarla JJ, Raven PH (1984) Pollen characters in relation to the delimitation of Myrtales. Ann Mo Bot Gard 71:858–969
Payne WW (1972) Observations of harmomegathy in pollen of anthophyta. Grana 12:93–98
Payne WW (1981) Structure and function in angiosperm pollen wall evolution. Rev Palaeobot Palynol 35:39–59
Punt W (1986) Functional factors influencing pollen form. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 97–101
Retief Ε, Van Wyk AE (1997) Palynology of southern African Boraginaceae: the genera Lobostemon, Echiostachys and Echium. Grana 36:271–278
Rowley JR, Skvarla JJ (2000) The elasticity of the exine. Grana 37:1–7
Thanikaimoni G (1986) Pollen apertures: form and function. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 119–136
Waha M, Hesse M (1986) Aperture types within Sapranthus and Polyalthia (Annonaceae). P1 Syst Evol 161:135–146
Walker JW, Doyle JA (1975) The bases of angiosperm phylogeny: palynology. Ann Missouri Bot Gard 62:664–723
Wodehouse RP (1935) Pollen grains. McGraw-Hill, New York 574 p
Acknowledgments
The authors are thankful to electron microscopy laboratory of Moscow State University. The manuscript was improved through helpful comments from reviewers. The study was supported by RFBR, research project No 13-04-00624 A.
Author information
Authors and Affiliations
Corresponding authors
Appendix
Appendix
Cryptantha celosioides (Eastw.) Payson (MHA) (1) Fremont Co. T 40 N R106W S14, SE EDGE of Trail Lake at inlet of torrey creek, CA 8.2 air Mi SSE of Dubois, CA 6.9 MI S ON trail lake road. Edge of meadow North of Greek. 27.07.1985. Elev. 7,400 ft. June Haines 5169 with georgia haines. Rocky mountain herbarium (RM). (2) Two miles NE of Omak, Okanogan Co. Rare on open rocky Sagebrush slopes at edge of valley; flowers white. No 16141. Col. J. A. Calder, J. A. Parmelee, R. L. Taylor. 8.05.1956. Cryptantha coryi I.M. Johnst. (MHA) Val Verde Co.: About ten miles east of Langtry Corolla white, turning orange with age. D. S. Correl and Helen B. Correl. No 30818. 3.04.1965. Heliotropium europaeum L. (MW) Tauricheskaya province, Berdyansk county, Girsovka village, Plowing-salt marshes. 18.07.1998. D. Duz. Myosotis palustris (L.) L. Botanical Garden of the Komarov Botanical Institute. 07.2010. Rindera bungei Gürke (MHA) USSR. Turkmenistan. Ashhabatskaya area. Central Kopetdag, Gopal-Dag, on the rocky substrate summit. 2,886 m above sea level. Coll. and Def. V. V. Nikitin. 06.25.1958. Rindera tetraspis Pall. (MHA) Uzbekistan, western spur of Zeravshanskogo ridge, env. per. Tyahtakaracha. Altitude of about 1,700 m, rocky slope. 27.04.1958. Col. A. K. Skvortsov, Def. N. Y. Stepanova. 10.06.2010.
Rights and permissions
About this article
Cite this article
Volkova, O.A., Severova, E.E. & Polevova, S.V. Structural basis of harmomegathy: evidence from Boraginaceae pollen. Plant Syst Evol 299, 1769–1779 (2013). https://doi.org/10.1007/s00606-013-0832-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0832-8