Abstract
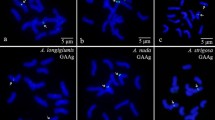
The genus Hypochaeris offers an excellent model for studies of recent adaptive radiation in the South American continent. We used karyotype analysis with chromomycin A3 (CMA3)/4’,6-diamidino-2-phenylindole (DAPI) banding and fluorescence in situ hybridization (FISH), and amplified fragment length polymorphism (AFLP) fingerprinting to investigate for the first time the Brazilian endemic H. catharinensis and define its position within the South American group of species. Strong CMA-positive signals were seen at the end of both arms of chromosome 3 and at the end of the long arm of chromosome 4. DAPI bands were only detected in subterminal position on short arm of chromosome 4. FISH with 5S and 35S ribosomal DNA (rDNA) probes revealed a single 5S rDNA locus on short arm of chromosome 2, typical for all other South American Hypochaeris taxa analyzed to date. The 35S rDNA locus was identified at subterminal position on the short arm of chromosome 3, as reported so far for only two of the known species (H. lutea and H. patagonica). The AFLP study included 55 individuals, comprising nine species of the South American Hypochaeris plus their putative ancestor H. angustifolia. Eleven AFLP primer combinations generated a total of 401 fragments, of which 388 (96.7%) were polymorphic. High genetic similarities were observed among taxa, with all South American Hypochaeris species falling into one main cluster [100% bootstrap (BS)]. Hypochaeris catharinensis is closely related to H. lutea (82% BS), forming a well-separated subcluster within the South American species. Taken together, the karyological and AFLP data contribute to the placement of H. catharinensis within the phylogenetic framework of South American species of Hypochaeris and allow the definition of a novel and well-resolved phylogenetic group (the Lutea group).


Similar content being viewed by others
References
Almeida JA (2009) Fatores Abióticos. In: Boldrini II (ed) Biodiversidade em campos do Planaltos de Araucária. Ministério do Meio Ambiente, Brasília, pp 19–38
Azevêdo-Gonçalves CF, Matzenbacher NI (2005) Taxonomic notes in Hypochaeris L. (Asteraceae). Compositae Newsl 42:1–4
Azevêdo-Gonçalves CF, Matzenbacher NI (2006) Notas Nomenclaturais em Hypochaeris L. (Asteraceae). Pesquisas Botânica 57:157–159
Azevêdo-Gonçalves CF, Matzenbacher NI (2007) O gênero Hypochaeris L. (Asteraceae) no Rio Grande do Sul, Brazil. Iheringia, Ser Bot 62:55–87
Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A (2006) Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439:719–723
Bortiri E (1999) Flora Fanerogamica Argentina. Asteraceae, Lactuceae: Hypochaeris. CONICET 63:1–25
Bussell JD, Waycott M, Chappill JA (2005) Arbitrarily amplified DNA markers as characters for phylogenetic inference. Perspect Plant Ecol 7:3–26
Cabrera AL (1937) Compuestas Argentinas nuevas o interessantes. Notas del Museo de la Plata 2:171–204
Cabrera AL (1963) Estudios sobre o gênero Hypochoeris. Boletín de la Sociedad Argentina de Botánica 10:166–195
Cabrera AL (1974) Compositae. In: Burkart A (ed) Flora Ilustrada de Entre Ríos (Argentina). Colleccion Científica del INTA, Buenos Aires, pp 512–525
Cabrera AL (1976) Materiales para una revisión del gênero Hypochoeris. I. Hypochoeris chillensis (H.B.K.) Hieron. Darwiniana 20:312–322
Cabrera AL, Crisci JV, Delucchi G, Freire SE, Giuliano DA, Iharlegui L, Katinas L, Sáenz AA, Sancho G, Urtubey E (2000) Catalogo ilustrado de las compuestas (Asteraceae) de la provincia de Buenos Aires, Argentina: Sistematica, Ecología y Usos. CONICET, Buenos Aires
Cerbah M, Coulaud J, Godelle B, Siljak-Yakovlev S (1995) Genome size, fluorochrome banding, and karyotype evolution in some Hypochaeris species. Genome 38:689–695
Cerbah M, Coulaud J, Siljak-Yakovlev S (1998a) rDNA organization and evolutionary relationship in the genus Hypochaeris (Asteraceae). J Hered 89:312–318
Cerbah M, Souza-Chies T, Jibier MF, Lejeune B, Siljak-Yakovlev S (1998b) Molecular phylogeny of the genus Hypochaeris using internal transcribed spacers of nuclear rDNA: inference for chromosomal evolution. Mol Biol Evol 15:345–354
Coelho ASG (2001) Software BOOD—Avaliação dos erros associados a estimativas de distâncias/similaridades genéticas através do procedimento de bootstrap com número variável de marcadores, v 1.1. Universidade Federal de Goiás, Goiânia
DeFillips RA (1976) Hypochaeris L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, Chater AO, Defilipps RA, Richardson IBK (eds) Flora Europea, vol 4. Cambridge University Press, Cambridge, pp 308–317
Doyle JJ, Doyle JL (1987) A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Felsenstein J (2004) Inferring phylogenies. Sinauer Associates, Massachusetts
Fernandes T, Rego LNAA, Nardy M, Yuyama PM, Vanzela ALL (2009) Karyotype differentiation of four Cestrum species (Solanaceae) revealed by fluorescent chromosome banding and FISH. Genet Mol Biol 32:320–327
Fiorin FG (2008) Número de cromossomos e estrutura dos cariótipos de duas espécies de Hypochaeris, (H. catharinensis e H. lutea) Asteraceae endêmicas do sul do Brasil. Dissertation, University of Londrina, Brazil
Fregonezi JN, Fernandes T, Torezan JMD, Vieira AO, Vanzela ALL (2006) Karyotype differentiation of four Cestrum species (Solanaceae) based on physical mapping of repetitive DNA. Genet Mol Biol 29:97–104
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Guerra M (2000) Patterns of heterochromatin distribution in plant chromosomes. Genet Mol Biol 23:1029–1041
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Koopman WJM (2005) Phylogenetic signal in AFLP data sets. Syst Biol 54:197–217
Koopman WJM, Wissemann V, Cock KD, Huylenbroeck JV, Riek JD, Sabatino GJH, Visser D, Vosman B, Ritz CM, Maes B, Werlemark G, Nybom H, Debener T, Linde M, Smulders MJM (2008) AFLP markers as a tool to reconstruct complex relationships: a case study in Rosa (Rosaceae). Am J Bot 95:353–366
Marcon AB, Barros IC, Gerra M (2004) Variation in chromosome numbers, CMA bands and 45S rDNA sites in species of Selaginella (Pteridophyta). Ann Bot 95:271–276
Matzenbacher NI (1998) O complexo “Senecionoide” (Asteraceae—Senecioneae) no Rio Grande do Sul—Brasil. Dissertation, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Mueller UG, Wolfenbarger LL (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Nardy M, Yuyama PM, Rego LNAA, Vanzela ALL (2010) Chromosome banding patterns and localization of 5S and 45S rDNA sites in three shrub-tree species of Erythrina L. (Leguminosae: Papilionoideae) from Brazil. Braz J Biosci 8:149–153
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Ortiz MA, Tremetsberger K, Talavera S, Stuessy TF, Garcia-Castano JL (2007) Population structure of Hypochaeris salzmanniana DC. (Asteraceae), an endemic species to the Atlantic coast on both sides of the Strait of Gibraltar, in relation to Quaternary sea level changes. Mol Ecol 16:541–552
Ortiz MA, Tremetsberger K, Terrab A, Stuessy TF, García-Castaño JL, Urtubey E, Baeza CM, Ruas CF, Gibbs PE, Talavera S (2008) Phylogeography of the invasive weed Hypochaeris radicata (Asteraceae): from Moroccan origin to worldwide introduced populations. Mol Ecol 17:3664–3667
Pellmyr O, Segraves KA, Althoff DM, Balcazar-Lara M (2007) The phylogeny of yuccas. Mol Phylogenet Evol 43:493–501
Ronquist F, Huelsenbeck JP, van der Mark P (2005) MrBayes 3.1 Manual draft 5/26/2005. School of Computational Science, Florida State University, Tallahassee
Ruas CF, Ruas PM, Matzenbacher NI, Ross G, Bernini C, Vanzela ALL (1995) Cytogenetic studies of some Hypochaeris species (Compositae) from Brazil. Am J Bot 82:369–375
Ruas CF, Vanzela ALL, Santos MO, Fregonezi JN, Ruas PM, Matzenbacher NI, Aguiar-Perecin ML (2005) Chromosomal organization and phylogenetic relationships in Hypochaeris species (Asteraceae) from Brazil. Genet Mol Biol 28:129–139
Ruas CF, Weiss-Schneeweiss H, Stuessy TF, Samuel MR, Pedrosa-Harand A, Tremetsberger K, Ruas PM, Schlüter PM, Herrera MAO, König C, Matzenbacher NI (2008) Characterization, genomic organization and chromosome distribution of Ty1-copia retrotransposons in species of Hypochaeris (Asteraceae). Gene 412:39–49
Samuel R, Stuessy TF, Tremetsberger K, Baeza CM, Siljak-Yakovlev S (2003) Phylogenetic relationships among species of Hypochaeris (Asteraceae, Cichorieae) based on ITS, plastid trnL intron, trnL-F spacer, and matK sequences. Am J Bot 90:496–507
Schubert I (1984) Mobile nucleolus organizing regions (NORs) in Allium (Liliaceae s lat)-Inferences from the specifity of silver staining. Plant Syst Evol 144:291–305
Silva CRM, González-Elizondo MS, Rego LNAA, Torezan JMD, Vanzela ALL (2008) Cytogenetical and cytotaxonomical analysis of some Brazilian species of Eleocharis (Cyperaceae). Aust J Bot 56:82–90
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Stuessy TF, Tremetsberger K, Müllner AN, Jankowicz J, Guo YP, Baeza CM, Samuel RM (2003) The melding of systematics and biogeography through investigations at the populational level: examples from the genus Hypochaeris (Asteraceae). Basic Appl Ecol 4:287–296
Stuessy T, Tremetsberger K, Samuel R, Jankowicz J, Guo YP, Muellner AN, Baeza CM (2004) Phylogenetic relationships among South American species of Hypochaeris (Asteraceae) based on AFLP data. In: Schaal BA, Chiang TY, Chuou CH (eds) Plant evolutionary genetics and the biology of weeds. Endemic Species Research Institute, Chi-Chi, pp 23–39
Swofford DL (2003) PAUP*: PHYLOGENETIC analysis using parsimony (* and other methods), v 4.0b10. Sinauer Associates, Sunderland
Terrab A, Ortiz MA, Talavera M, Ariza MJ, Moriana MC, Garrcía-Cataño JL, Tremetsberger K, Stuessy TF, Baeza M, Urtubey E, Ruas CF, Casimiro-Soringuer R, Balao F, Gibbs PE, Talavera S (2009) AFLP and breeding system studies indicate vicariance origin for scattered populations and enigmatic low fecundity in the Moroccan endemic Hypochaeris angustifolia (Asteraceae), sister taxon to all of the South American Hypochaeris species. Mol Phylogenet Evol 53:13–22
Tremetsberger K, Stuessy TF, Samuel RM, Baeza CM, Fay M (2003) Genetics of colonization in Hypochaeris tenuifolia (Asteraceae, Lactuceae) on Volcán Lonquimay Chile. Mol Ecol 12:2649–2659
Tremetsberger K, Weiss-Schneeweiss H, Stuessy T, Samuel R, Kadlec G, Ortiz MA, Talavera S (2005) Nuclear ribosomal DNA and karyotypes indicate a NW African origin of South American Hypochaeris (Asteraceae, Cichorieae). Mol Phylogenet Evol 35:102–116
Tremetsberger K, Stuessy TF, Kadlec G, Urtubey E, Baeza CM, Beck SG, Valdebenito HA, Ruas CF, Matzenbacher NI (2006) AFLP phylogeny of South American species of Hypochaeris (Asteraceae, Lactuceae). Syst Bot 3:610–626
Vanzela ALL, Ruas CF, Oliveira MF, Ruas PM (2002) Characterization of diploid, tetraploid and hexaploid Helianthus species by chromosome banding and FISH with 45S rDNA probe. Genetica 114:105–111
Vos P, Hogers R, Bleeker M, Reijans M, Van DE, Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acid Res 23:4407–4414
Weiss H, Stuessy TF, Grau J, Baeza CM (2003) Chromosome reports from South American Hypochaeris (Asteraceae). Ann Mo Bot Gard 90:56–63
Weiss-Schneeweiss H, Stuessy TF, Siljak-Yakovlev S, Baeza CM, Parker J (2003) Karyotype evolution in South American species of Hypochaeris (Asteraceae, Lactuceae). Plant Syst Evol 241:171–184
Weiss-Schneeweiss H, Stuessy TF, Tremetsberger K, Urtubey E, Valdebenito HA, Beck SG, Baeza CM (2007) Chromosome numbers and karyotypes of South American species and populations of Hypochaeris (Asteraceae). Bot J Linn Soc 153:49–60
Weiss-Schneeweiss H, Tremetsberger K, Schneeweiss GM, Parker JS, Stuessy TF (2008) Karyotype diversifications and evolution in diploid and polyploid South American Hypochaeris (Asteraceae) inferred from rDNA localization and genetic fingerprint data. Ann Bot 101:909–918
Acknowledgments
We sincerely thank The Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP6510), the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grants to C.F.R. and P.M.R. CNPQ no. 201254/2003-4 and 201332/2003-5), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes, and Fundación BBVA for providing funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reck, M., Benício, L.M., Ruas, E.A. et al. Karyotype and AFLP data reveal the phylogenetic position of the Brazilian endemic Hypochaeris catharinensis (Asteraceae). Plant Syst Evol 296, 231–243 (2011). https://doi.org/10.1007/s00606-011-0490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0490-7