Abstract
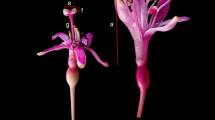
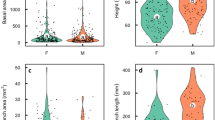
Anthonotha noldeae (Rossberg) Exell et Hillc. Leguminosae, Caesalpinioideae, an Afromontane forest canopy tree, produces a superabundance of flowers, but few (1.1%) initiate fruits, and even fewer (0.44%) reach maturity and disperse. Fifty-three percent of fruits are predated. A. noldeae is andromonoecious. Over all 20 trees for which we counted floral gender ratios (hermaphrodite vs. staminate flowers), the ratio was 2.1:1. There was significant (F19,379 = 8.52; p < 0.001) variation in the proportion of the two flower types produced among individuals. Floral gender ratios did not predict the proportion of mature fruits produced on a tree (r2 = 0.066; p = 0.273), or its phenotypic gender (Gi)—which is defined as the contribution of an individual tree to the next generation in terms of female function relative to all other individuals in the population (r2 = 0.069; p = 0.262). Gi varied among trees from 0 (functionally male) to 0.8. There was no correlation between tree size and phenotypic gender (r = 0.08; p = 0.74; n = 20). Open flowers initiated significantly more fruits than bagged or caged flowers (p = 0.0073), showing A. noldeae relies on birds to produce fruits. However, 80% of visits to flowers were made by insects, while only 20% of visits were from pollinating sunbirds. While low fruit initiation was the most restrictive reproductive step, low pollination rates combined with insect robbers, fungal attacks and high levels of predation of immature fruits may contribute to the extremely low fruit set in A. noldeae.



Similar content being viewed by others
References
Agmen FL, Chapman HM, Bawuro M (2009) Seed dispersal by tantalus monkeys Chlorocebus tantalus tantalus) in a Nigerian montane forest. Afr J Ecol. doi:10.1111/j.1365-2028.2009.01190.x
Ashman TL (2002) The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology 83:1175–1184
Barrett Harder (2007) David G. Lloyd and the evolution of floral biology: from natural history to strategic analysis. In: Harder LD, Barrett SCH (eds) Ecology and evolution of flowers. Oxford University Press, New York
Bawa KS, Webb CJ (1984) Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Amer J Bot 71:736–751
Bertin RI (1982) The ecology of sex expression in Red Buckeye. Ecology 63:445–456
Bierzychudek P (1981) Pollinator limitation of plant reproductive effort. Am Nat 117:838–840
Breteler FJ (2008) Anthonotha and Isomacroobium (Leguminosae, Caesalpinioideae): two distinct genera. Syst Geogr Plants 78:137–144
Breteler FJ (2010) Revision of the African genus Anthonotha (Leguminosae, Caesalpinioideae). Plant Ecol Evol 143(1):70–99
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Burd M (1998) “Excess” flower production and selective fruit abortion: a model of potential benefits. Ecology 79(6):2123–2132
Chapman JD, Chapman HM (2001) The forests of Taraba and Adamawa States, Nigeria: an ecological account and plant species checklist. University of Canterbury, Christchurch
Charnov EL (1982) Parent-offspring conflict over reproductive effort. Am Nat 119(5):736–737
Cuevas J, Polito VS (2004) The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind-pollinated taxon. Ann Bot 93:547–553
Diggle PI (1991) Labile sex expression in andromonoecious Solanum hirtum: floral development and sex determination. Amer J Bot 78:377–393
Diggle PK (1994) The expression of andromonoecy in Solanum hirsutum (Solanaceae): phenotypic plasticity and ontogenetic contingency. Amer J Bot 81:1334–1365
Dudash M (1991) Plant size effects on male and female function. Ecology 72:1004–1012
Ehlren J (1991) Why do plants produce surplus flowers? A reserve-ovary model. Am Nat 138(4):918–933
Gawaisa S (2010) Feeding behaviour of the putty nose monkey, Circopithecus nictitans. Dissertation Federal University of Technology, Yola
Gibbs PE, Lewis GP, Lughadha EN (1999) Fruit-set induced changes in the sex of flowers in Caesalpinia calycina (Leguminosae). Plant Biol 1(6):665–669
Hokche O, Ramirez N (1990) Pollination ecology of seven species of Bauhinia L. (Leguminosae: Caesalpinioidea). Ann Mo Bot Gard 77:559–572
Janzen DH (1977) A note on optimal mate selection by plants. Am Nat 111:365–371
Klinkhamer PGL, de Jong TJ, Metz H (1997) Sex and size in cosexual plants. Trends Ecol Evol 12:260–265
Lewis G, Gibbs P (1999) Reproductive biology of Caesalpinia calycina and C. pluviosa (Leguminosae) of the caatinga of north-eastern Brazil. Plant Syst Evol 217:43–53
Lloyd DG (1980a) Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive season. New Phytol 86:69–79
Lloyd DG (1980b) Sexual strategies in plants. III. A quantitative method for describing the gender of plants. N Z J Bot 18:103–108
Lloyd DB, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evol Biol 17:255–338
Lovett-Doust J (1980) Floral sex ratios in andromonoecious Umbelliferae. New Phytol 85:265–273
Marshall DL, Ellstrand NC (1988) Effective mate choice in wild radish: evidence for selective seed abortion and its mechanism. Am Nat 131(5):739–756
Mendez M (1998) Modification of phenotypic and functional gender in the monoecious Arum italicum (Araceae). Am J Bot 85(2):225–234
Miller JS, Diggle PK (2003) Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. Am J Bot 90(5):707–715
Miller JS, Diggle PK (2007) Correlated evolution of fruit size and sexual expression in andromonoecious Solanum sections Acanthophora and Lasiocarpa (Solanaceae). Am J Bot 94(10):1706–1715
Primack RB, Lloyd DG (1980) Sexual strategies in plants IV. The distributions of gender in two monomorphic shrub populations. NZ J Bot 18:109–114
Quesada-Aguilar A, Kalisz S, Ashman TL (2008) Flower morphology and pollinator dynamics in Solanum carolinense (Solanaceae): implications for the evolution of andromonoecy. Am J Bot 95:974–984
Ramirez N, Sobrevila C, de Enrech NX, Ruiz-Zapata T (1984) Floral biology and breeding system of Bauhinia benthamiana Taub. (Leguminosae), a bat-pollinated tree in Venezuelan “Llanos”. Am J Bot 71(2):273–280
Sarkissian TS, Barrett SCH, Harder LD (2001) Gender variation in Sagittaria Latifolia (Alismataceae): is size all that matters? Ecology 82(2):360–373
Solomon BP (1986) Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae). Am J Bot 73(8):1215–1221
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Annu Rev Ecol Syst 12:253–279
Sutherland S, Delph LF (1984) On the importance of male fitness in plants: patterns of fruit-set. Ecology 65:1093–1104
Vallejo-Marin M, Rausher MD (2006) The role of male flowers in andromonoecious species: energetic costs and siring success in Solanum carolinense L. Evolution 60:404–412
Weston KA (2009) Mistletoe reproductive mutualisms in a West African montane forest. Dissertation, University of Canterbury
Whalen MD, Costich DE (1986) Andromonoecy in Solanum. In: D’Arcy WGD (ed) Solanaceae: biology and systematics. Columbia University Press, New York, pp 285–302
Wise MJ, Cummins JJ (2007) Herbivory as an agent of natural selection for floral-sex ratio in horsenettle (Solanum carolinense). Evol Ecol Res 9(8):1319–1328
Wise MJ, Hebert JB (2010) Herbivores affect natural selection for floral-sex ratio in a field population of horsenettle, Solanum carolinense. Ecology 91:937–943
Acknowledgments
We thank the Nigerian Conservation Foundation for their invitation to conduct field work, the Taraba State Forest Service for our research site, Charles Nsor and Kennedy Poloma for pollinator observations, and Augustine Ntim for field assistance. This manuscript benefited greatly from discussions with Spencer Barrett and Colin Webb, as well as anonymous reviewers. This research was funded by The North of England Zoological Society, the A.P. Leventis Foundation and Nexen Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beavon, M.A., Chapman, H.M. Andromonoecy and high fruit abortion in Anthonotha noldeae in a West African montane forest. Plant Syst Evol 296, 217–224 (2011). https://doi.org/10.1007/s00606-011-0488-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0488-1