Abstract
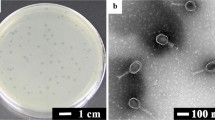
A broad host range phage-based nanozyme (Fe-MOF@SalmpYZU47) was prepared for colorimetric detection of multiple Salmonella enterica strains. The isolation of a broad host range phage (SalmpYZU47) capable of infecting multiple S. enterica strains was achieved. Then, it was directly immobilized onto the Fe-MOF to prepare Fe-MOF@SalmpYZU47, exhibiting peroxidase-like activity. The peroxidase-like activity can be specifically inhibited by multiple S. enterica strains, benefiting from the broad host range capture ability of Fe-MOF@SalmpYZU47. Based on it, a colorimetric detection approach was developed for S. enterica in the range from 1.0 × 102 to 1.0 × 108 CFU mL−1, achieving a low limit of detection (LOD) of 11 CFU mL−1. The Fe-MOF@SalmpYZU47 was utilized for detecting S. enterica in authentic food samples, achieving recoveries ranging from 91.88 to 105.34%. Hence, our proposed broad host range phage-based nanozyme exhibits significant potential for application in the colorimetric detection of pathogenic bacteria.
Graphical Abstract







Similar content being viewed by others
References
Bearson S (2022) Salmonella in swine: prevalence, multidrug resistance, and vaccination strategies. Annu Rev Animal Biosci 10:373–393. https://doi.org/10.1146/annurev-animal-013120-043304
Guan L, Hu A, Ma S, Liu J, Yao X, Ye T, Han M, Yang C, Zhang R, Xiao X, Wu Y (2024) Lactiplantibacillus plantarum postbiotic protects against Salmonella infection in broilers via modulating NLRP3 inflammasome and gut microbiota. Poult Sci 103(4):103483–103483. https://doi.org/10.1016/j.psj.2024.103483
Jennings E, Thurston T, Holden D (2017) Salmonella SPI-2 type III secretion system effectors: molecular mechanisms and physiological consequences. Cell Host Microbe 22(2):217–231. https://doi.org/10.1016/j.chom.2017.07.009
Collier S, Deng L, Adam E, Benedict K, Beshearse E, Blackstock A, Bruce B, Derado G, Edens C, Fullerton K, Gargano J, Geissler A, Hall A, Havelaar A, Hill V, Hoekstra R, Reddy S, Scallan E, Stokes E, Yoder J, Beach M (2021) Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg Infect Dis 27(1):140–149. https://doi.org/10.3201/eid2701.190676
Vinayaka A, Ngo T, Kant K, Engelsmann P, Dave V, Shahbazi M, Wolff A, Bang D (2019) Rapid detection of Salmonella enterica in food samples by a novel approach with combination of sample concentration and direct PCR. Biosens Bioelectron 129:224–230. https://doi.org/10.1016/j.bios.2018.09.078
Jiao C, Duan W, Wu X, Shang Y, Zhang F, Zhang M, Chen X, Zeng J, Yang C (2023) Multifunctional nanoprobe-amplified enzyme-linked immunosorbent assay on capillary: a universal platform for simple, rapid, and ultrasensitive dual-mode pathogen detection. Anal Chem 95(30):11316–11325. https://doi.org/10.1021/acs.analchem.3c01375
Castle L, Schuh D, Reynolds E, Furst A (2021) Electrochemical sensors to detect bacterial foodborne pathogens. Acs Sensors 6(5):1717–1730. https://doi.org/10.1021/acssensors.1c00481
Hussain W, Ullah M, Farooq U, Aziz A, Wang S (2021) Bacteriophage-based advanced bacterial detection: concept, mechanisms, and applications. Biosens Bioelectron 177:112973–112973. https://doi.org/10.1016/j.bios.2021.112973
Qin L, Xiao J, Yang H, Liang J, Li L, Wu S, Peng D (2024) Rapid immunoassays for the detection of quinoxalines and their metabolites residues in animal-derived foods: a review. Food Chem 443:138539–138539. https://doi.org/10.1016/j.foodchem.2024.138539
Ma S, Luo X, Kong J, Li X, Cao Z, Wang X, Cai W, Wang L, Ran G (2022) Plasmonic silver loaded hybrid Bi-Ag nanoalloys for highly efficient disinfection by enhancing photothermal performance and interface capability. Chem Eng J 450:138016. https://doi.org/10.1016/j.cej.2022.138016
Zhao J, Han M, Ma A, Jiang F, Chen R, Dong Y, Wang X, Ruan S, Chen Y (2024) A machine vision-assisted Argonaute-mediated fluorescence biosensor for the detection of viable Salmonella in food without convoluted DNA extraction and amplification procedures. J Hazard Mater 466:133648–133648. https://doi.org/10.1016/j.jhazmat.2024.133648
Qi W, Zheng L, Hou Y, Duan H, Wang L, Wang S, Liu Y, Li Y, Liao M, Lin J (2022) A finger-actuated microfluidic biosensor for colorimetric detection of foodborne pathogens. Food Chem 381:131801–131801. https://doi.org/10.1016/j.foodchem.2021.131801
Lin X, Zhao M, Peng T, Zhang P, Shen R, Jia Y (2023) Detection and discrimination of pathogenic bacteria with nanomaterials-based optical biosensors: a review. Food Chem 426:136578–136578. https://doi.org/10.1016/j.foodchem.2023.136578
Zhang X, Lin S, Liu S, Tan X, Dai Y, Xia F (2021) Advances in organometallic/organic nanozymes and their applications. Coord Chem Rev 429:213652. https://doi.org/10.1016/j.ccr.2020.213652
Feng M, Li X, Zhang X, Huang Y (2023) Recent advances in the development and analytical applications of oxidase-like nanozymes. Trac-Trends Anal Chem 166:117220. https://doi.org/10.1016/j.trac.2023.117220
Niu K, Chen J, Lu X (2023) Versatile biomimetic catalyst functionalized nanozymes for electrochemical sensing. Chem Eng J 475:146491. https://doi.org/10.1016/j.cej.2023.146491
Zhao Y, Wang X, Pan S, Hong F, Lu P, Hu X, Jiang F, Wu L, Chen Y (2024) Bimetallic nanozyme-bioenzyme hybrid material-mediated ultrasensitive and automatic immunoassay for the detection of aflatoxin B1 in food. Biosens Bioelectron 248:115992. https://doi.org/10.1016/j.bios.2023.115992
Zhu S, Tang Y, Shi B, Zou W, Wang X, Wang C, Wu Y (2021) Oligonucleotide-mediated the oxidase-mimicking activity of Mn3O4 nanoparticles as a novel colorimetric aptasensor for ultrasensitive and selective detection of Staphylococcus aureus in food. Sens Actuators B-Chem 349:130809. https://doi.org/10.1016/j.snb.2021.130809
Costa S, Nogueira C, Cunha A, Lisac A, Carvalho C (2023) Potential of bacteriophage proteins as recognition molecules for pathogen detection. Crit Rev Biotechnol 43(5):787–804. https://doi.org/10.1080/07388551.2022.2071671
Xu X, Xu Q, Li W, Xiao F, Xu H (2024) From engineered photoactive materials to detection signal amplification strategies in photoelectrochemical biosensing of pathogens: new horizons and perspectives. Chem Eng J 480:147941. https://doi.org/10.1016/j.cej.2023.147941
Hatfull G, Dedrick R, Schooley R (2022) Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 73:197–211. https://doi.org/10.1146/annurev-med-080219-122208
Strathdee S, Hatfull G, Mutalik V, Schooley R (2023) Phage therapy: from biological mechanisms to future directions. Cell 186(1):17–31. https://doi.org/10.1016/j.cell.2022.11.017
Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, Spriet I, Depypere M, Wagemans J, Lavigne R, Pirnay J, Merabishvili M, Munter P, Peetermans W, Dupont L, Gerven L, Metsemakers W (2022) Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect Dis 22(8):E208–E220. https://doi.org/10.1016/s1473-3099(21)00612-5
Huang C, Zhao J, Lu R, Wang J, Nugen S, Chen Y, Wang X (2023) A phage-based magnetic relaxation switching biosensor using bioorthogonal reaction signal amplification for Salmonella detection in foods. Food Chem 400:134035–134035. https://doi.org/10.1016/j.foodchem.2022.134035
Ye J, Guo J, Li T, Tian J, Yu M, Wang X, Majeed U, Song W, Xiao J, Luo Y, Yue T (2022) Phage-based technologies for highly sensitive luminescent detection of foodborne pathogens and microbial toxins: a review. Compr Rev Food Sci Food Safety 21(2):1843–1867. https://doi.org/10.1111/1541-4337.12908
Zhou Y, Marar A, Kner P, Ramasamy R (2017) Charge-directed immobilization of bacteriophage on nanostructured electrode for whole-cell electrochemical biosensors. Anal Chem 89(11):5735–5742. https://doi.org/10.1021/acs.analchem.6b03751
Gao L, Ouyang M, Li Y, Zhang H, Zheng X, Li H, Rao S, Yang Z, Gao S (2022) Isolation and characterization of a lytic vibriophage OY1 and its biocontrol effects against Vibrio spp. Front Microbiol 13:830692–830692. https://doi.org/10.3389/fmicb.2022.830692
Rai S, Tyagi A, Kumar B, Reddy S (2023) Isolation and characterization of Aeromonas hydrophila lytic phage, and evaluation of a phage cocktail against A. hydrophila contamination in fish fillet. Food Control 145:109460. https://doi.org/10.1016/j.foodcont.2022.109460
Denyes J, Dunne M, Steiner S, Mittelviefhaus M, Weiss A, Schmidt H, Klumpp J, Loessner M (2017) Modified bacteriophage S16 long tail fiber proteins for rapid and specific immobilization and detection of Salmonella cells. Appl Environ Microbiol 83(12):E00277-E317. https://doi.org/10.1128/aem.00277-17
Wang X, Yun Y, Sun W, Lu Z, Tao X (2022) A high-performance fluorescence immunoassay based on pyrophosphate-induced MOFs NH2-MIL-88B(Fe) hydrolysis for chloramphenicol detection. Sens Actuators B-Chem 353:131143. https://doi.org/10.1016/j.snb.2021.131143
Darabdhara G, Sharma B, Das M, Boukherroub R, Szunerits S (2017) Cu-Ag bimetallic nanoparticles on reduced graphene oxide nanosheets as peroxidase mimic for glucose and ascorbic acid detection. Sens Actuators B-Chem 238:842–851. https://doi.org/10.1016/j.snb.2016.07.106
Wei J, Chen X, Shi S, Mo S, Zheng N (2015) An investigation of the mimetic enzyme activity of two-dimensional Pd-based nanostructures. Nanoscale 7(45):19018–19026. https://doi.org/10.1039/c5nr05675f
Zhang Y, Xu X, Yang J, Tan M, Zhou W, Ga L, Yang Z (2023) Directional immobilization of phage on the palladium-based nanozyme for colorimetric detection of Cronobacter sakazakii in powdered infant formula. LWT-Food Sci Technol 186:115260. https://doi.org/10.1016/j.lwt.2023.115260
Zhou W, Wen H, Hao G, Zhang Y, Yang J, Gao L, Zhu G, Yang Z, Xu X (2023) Surface engineering of magnetic peroxidase mimic using bacteriophage for high-sensitivity/specificity colorimetric determination of Staphylococcus aureus in food. Food Chem 426:136611–136611. https://doi.org/10.1016/j.foodchem.2023.136611
Arnaud C, Effantin G, Vives C, Engilberge S, Bacia M, Boulanger P, Girard E, Schoehn G, Breyton C (2017) Bacteriophage T5 tail tube structure suggests a trigger mechanism for Siphoviridae DNA ejection. Nat Commun 8:1953. https://doi.org/10.1038/s41467-017-02049-3
Ren Y, Wei J, Wang Y, Wang P, Ji Y, Liu B, Wang J, González-Sapienza G, Wang Y (2022) Development of a streptavidin-bridged enhanced sandwich ELISA based on self-paired nanobodies for monitoring multiplex Salmonella serogroups. Anal Chim Acta 1203:339705–339705. https://doi.org/10.1016/j.aca.2022.339705
Feng K, Li T, Ye C, Gao X, Yang T, Liang X, Yue X, Ding S, Dong Q, Yang M, Xiong C, Huang G, Zhang J (2021) A label-free electrochemical immunosensor for rapid detection of Salmonella in milk by using CoFe-MOFs-graphene modified electrode. Food Control 130:108357. https://doi.org/10.1016/j.foodcont.2021.108357
Zhou C, Zou H, Li M, Sun C, Ren D, Li Y (2018) Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO. Biosens Bioelectron 117:347–353. https://doi.org/10.1016/j.bios.2018.06.005
Yang M, Chen X, Zhu L, Lin S, Li C, Li X, Huang K, Xu W (2021) Aptamer-functionalized DNA-silver nanocluster nanofilm for visual detection and elimination of bacteria. ACS Appl Mater Interfaces 13(32):38647–38655. https://doi.org/10.1021/acsami.1c05751
Funding
This study is supported by the Natural Science Foundation of the Open Research Fund of Key Laboratory of Healthy Freshwater Aquaculture, Ministry of Agriculture and Rural Affairs (ZJK202314), the Natural Science Foundation of Jiangsu Province (BK20230586), the Natural Science Foundation of Yangzhou City (SZR2023000023), and the Higher Education Institutions of Jiangsu Province (23KJB550011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This research did not involve human or animal samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, L., Zhang, L., Yang, J. et al. Immobilization of a broad host range phage on the peroxidase-like Fe-MOF for colorimetric determination of multiple Salmonella enterica strains in food. Microchim Acta 191, 331 (2024). https://doi.org/10.1007/s00604-024-06402-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06402-4