Abstract
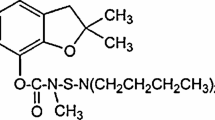
A cutting-edge electrochemical method is presented for precise quantification of amitraz (AMZ), a commonly used acaricide in veterinary medicine and agriculture. Leveraging a lab-made screen-printed carbon electrode modified with a synergistic blend of perylene tetracarboxylic acid (PTCA), mesoporous carbon (MC), and Nafion, the sensor’s sensitivity was significantly improved. Fine-tuning of PTCA, MC, and Nafion ratios, alongside optimization of the pH of the supporting electrolyte and accumulation time, resulted in remarkable sensitivity enhancements. The sensor exhibited a linear response within the concentration range 0.01 to 0.70 μg mL-1, boasting an exceptionally low limit of detection of 0.002 μg mL-1 and a limit of quantification of 0.10 μg mL-1, surpassing maximum residue levels permitted in honey, tomato, and longan samples. Validation with real samples demonstrated high recoveries ranging from 80.8 to 104.8%, with a relative standard deviation below 10%, affirming the method’s robustness and precision. The modified PTCA/MC/Nafion@SPCE-based electrochemical sensor not only offers superior sensitivity but also simplicity and cost-effectiveness, making it a pivotal tool for accurate AMZ detection in food samples. Furthermore, beyond the scope of this study, the sensor presents promising prospects for wider application across various electrochemical analytical fields, thereby significantly contributing to food safety and advancing agricultural practices.
Graphical Abstract









Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
References
Nováková K, Hrdlička V, Navrátil T, Harvila M, Zima J, Barek J (2016) Application of silver solid amalgam electrode for determination of formamidine amitraz. Monatshefte für Chemie - Chemical Monthly 147:181–9. https://doi.org/10.1007/s00706-015-1575-8
Iken I, Abdessadek M, El Attari A, Achour S (2020) Poisoning by amitraz, uncommon pesticide revealed by high performance liquid chromatography: about two cases. Toxicol Anal Clin 32:200–4. https://doi.org/10.1016/j.toxac.2019.12.001
Tokman N, Soler C, Farre M, Pico Y, Barcelo D (2009) Determination of amitraz and its transformation products in pears by ethyl acetate extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:3138–46. https://doi.org/10.1016/j.chroma.2009.01.099
Juan-Borrás M, Domenech E, Escriche I (2016) Mixture-risk-assessment of pesticide residues in retail polyfloral honey. Food Control 67:127–34. https://doi.org/10.1016/j.foodcont.2016.02.051
Mukherjee I (2009) Determination of pesticide residues in honey samples. Bull Environ Contam Toxicol 83:818–21. https://doi.org/10.1007/s00128-009-9772-y
Kubiak A, Biesaga M (2020) Solid phase-extraction procedure for the determination of amitraz degradation products in honey. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 37:1888–96. https://doi.org/10.1080/19440049.2020.1818850
Jimenez JJ, Nozal MJ, Bernal JL, Santos M, Mayorga AL (2002) Factors affecting the extraction, hydrolysis and derivatization steps for the quantitation of total residues of amitraz in honey by gas chromatography with electron capture detection. Anal Bioanal Chem 374:300–4. https://doi.org/10.1007/s00216-002-1475-8
Li P, Teng Y, Nie Y, Liu W (2017) SERS detection of insecticide amitraz residue in milk based on Au@Ag core-shell nanoparticles. Food Anal Methods 11:69–76. https://doi.org/10.1007/s12161-017-0966-3
Shamsipur M, Hassan J, Salar-Amoli J, Yamini Y (2008) Headspace solvent microextraction-gas chromatographic thermionic specific detector determination of amitraz in honey after hydrolysis to 2,4-dimethylaniline. J Food Compos Anal 21:264–70. https://doi.org/10.1016/j.jfca.2007.10.004
Lerdsri J, Upan J, Jakmunee J (2022) Nafion mixed carbon nanotube modified screen-printed carbon electrode as a disposable electrochemical sensor for quantification of Amitraz in honey and longan samples. Electrochim Acta 410. https://doi.org/10.1016/j.electacta.2022.140050
Xu JZ, Miao JJ, Lin H, Ding T, Zhao ZY, Wu B et al (2009) Determination of amitraz and 2,4-dimethylaniline residues in honey by using LC with UV detection and MS/MS. J Sep Sci 32:4020–4. https://doi.org/10.1002/jssc.200900437
Caldow M, Fussell RJ, Smith F, Sharman M (2007) Development and validation of an analytical method for total amitraz in fruit and honey with quantification by gas chromatography-mass spectrometry. Food Addit Contam 24:280–4. https://doi.org/10.1080/02652030601013638
Jiménez JJ, Bernal JL, del Nozal MJ, Alonso C (2004) Extraction and clean-up methods for the determination of amitraz total residues in beeswax by gas chromatography with electron capture detection. Anal Chim Acta 524:271–8. https://doi.org/10.1016/j.aca.2004.03.039
Taccheo MB, De Paoli M, Spessotto C (1988) Determination of total amitraz residue in honey by electron capture capillary gas chroma tography-a simplified method. Pestic Sci 23:59–64. https://doi.org/10.1002/ps.2780230108
Scholz F (2015) Voltammetric techniques of analysis: the essentials. ChemTexts 1. https://doi.org/10.1007/s40828-015-0016-y
Suresh RR, Lakshmanakumar M, Arockia Jayalatha J, Rajan K, Sethuraman S, Krishnan UM, et al. (2021) Fabrication of screen-printed electrodes: opportunities and challenges. 56:8951–9006. https://doi.org/10.1007/s10853-020-05499-1
Taleat Z, Khoshroo A, Mazloum-Ardakani MJMA (2014) Screen-printed electrodes for biosensing: a review (2008–2013). 181:865–91. https://doi.org/10.1007/s00604-014-1181-1
Krepper G, Pistonesi MF, Di Nezio MS (2019) Adsorptive square wave voltammetric determination of amitraz in Argentine honeys with a microwave-assisted sample treatment. Microchem J 150. https://doi.org/10.1016/j.microc.2019.104068
Wen C-Y, Chen J, Li M, Xue Y, Aslam S, Subhan F et al (2017) Gold nanoparticles deposited on mesoporous carbon as a solid-phase sorbent with enhanced extraction capacity and selectivity for anilines. Microchim Acta 184:3929–36. https://doi.org/10.1007/s00604-017-2415-9
Zhang Q, Zhang C, Ying Y, Ping J (2021) An easy-fabricated ordered mesoporous carbon-based electrochemical sensor for the analysis of trans-resveratrol in red wines. Food Control 129. https://doi.org/10.1016/j.foodcont.2021.108203
Regiart M, Magallanes JL, Barrera D, Villarroel-Rocha J, Sapag K, Raba J et al (2016) An ordered mesoporous carbon modified electrochemical sensor for solid-phase microextraction and determination of triclosan in environmental samples. Sens Actuators B: Chem 232:765–72. https://doi.org/10.1016/j.snb.2016.04.031
Zhang J, Li W, Zhu W, Yang Y, Qin P, Zhou Q et al (2019) Mesoporous graphitic carbon nitride as an efficient sorbent for extraction of sulfonamides prior to HPLC analysis. Microchim Acta 186:279. https://doi.org/10.1007/s00604-019-3394-9
Amara U, Mahmood K, Riaz S, Nasir M, Hayat A, Hanif M, et al. (2021) Self-assembled perylene-tetracarboxylic acid/multi-walled carbon nanotube adducts based modification of screen-printed interface for efficient enzyme immobilization towards glucose biosensing. Microchem J 165. https://doi.org/10.1016/j.microc.2021.106109
Amara U, Mehran MT, Sarfaraz B, Mahmood K, Hayat A, Nasir M et al (2021) Perylene diimide/MXene-modified graphitic pencil electrode-based electrochemical sensor for dopamine detection. Mikrochim Acta 188:230. https://doi.org/10.1007/s00604-021-04884-0
Yang X, Niu X, Mo Z, Guo R, Liu N, Zhao P et al (2019) Perylene-functionalized graphene sheets modified with chitosan for voltammetric discrimination of tryptophan enantiomers. Mikrochim Acta 186:333. https://doi.org/10.1007/s00604-019-3442-5
Zhu G, Yi Y, Han Z, Liu J, Gai Z (2014) 3,4,9,10-perylene tetracarboxylic acid noncovalently modified multiwalled carbon nanotubes: synthesis, characterization, and application for electrochemical determination of 2-aminonaphthalene. Anal Lett 47:2370–83. https://doi.org/10.1080/00032719.2014.905951
Ran X, Yang L, Zhang J, Deng G, Li Y, Xie X et al (2015) Highly sensitive electrochemical sensor based on beta-cyclodextrin-gold@3, 4, 9, 10-perylene tetracarboxylic acid functionalized single-walled carbon nanohorns for simultaneous determination of myricetin and rutin. Anal Chim Acta 892:85–94. https://doi.org/10.1016/j.aca.2015.08.046
Zhu G, Zhang X, Gai P, Zhang X, Chen J (2012) β-Cyclodextrin non-covalently functionalized single-walled carbon nanotubes bridged by 3,4,9,10-perylene tetracarboxylic acid for ultrasensitive electrochemical sensing of 9-anthracenecarboxylic acid. Nanoscale 4:5703–9. https://doi.org/10.1039/c2nr31378b
Ören T, Birel Ö, Anık Ü (2018) Electrochemical determination of dopamine using a novel perylenediimide-derivative modified carbon paste electrode. Anal Lett 51:1680–93. https://doi.org/10.1080/00032719.2017.1388816
Kludský M, Vopička O, Matějka P, Hovorka Š, Friess K (2018) Nafion® modified with primary amines: chemical structure, sorption properties and pervaporative separation of methanol-dimethyl carbonate mixtures. Eur Polym J 99:268–76. https://doi.org/10.1016/j.eurpolymj.2017.12.028
Chuntib P, Themsirimongkon S, Saipanya S, Jakmunee J (2017) Sequential injection differential pulse voltammetric method based on screen printed carbon electrode modified with carbon nanotube/Nafion for sensitive determination of paraquat. Talanta 170:1–8. https://doi.org/10.1016/j.talanta.2017.03.073
Silva TA, Moraes FC, Janegitz BC, Fatibello-Filho O (2017) Electrochemical biosensors based on nanostructured carbon black: a review. J Nanomater 2017:1–14. https://doi.org/10.1155/2017/4571614
Reanpang P, Upan J, Jakmunee J (2021) A novel flow injection amperometric sensor based on carbon black and graphene oxide modified screen-printed carbon electrode for highly sensitive determination of uric acid. Talanta 232:122493. https://doi.org/10.1016/j.talanta.2021.122493
Zeng J, Chen J, Song X, Wang Y, Ha J, Chen X et al (2010) An electrochemically enhanced solid-phase microextraction approach based on a multi-walled carbon nanotubes/Nafion composite coating. J Chromatogr A 1217:1735–41. https://doi.org/10.1016/j.chroma.2010.01.034
Moraes FC, Mascaro LH, Machado SA, Brett CM (2009) Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta 79:1406–11. https://doi.org/10.1016/j.talanta.2009.06.013
Jeevan RJ, Chandrasekar R, Bhaskar M, Radhakrishana G (2001) Separation of xylidine isomers by micellar electrokinetic chromatography. J Chromatogr Sci 39:332–8. https://doi.org/10.1093/chromsci/39.8.332
Poza-Nogueiras V, Pazos M, Sanromán MÁ, González-Romero E (2019) Double benefit of electrochemical techniques: Treatment and electroanalysis for remediation of water polluted with organic compounds. Electrochimica Acta 320. https://doi.org/10.1016/j.electacta.2019.134628
Planes GA, Rodríguez JL, Miras MC, García G, Pastor E, Barbero CA (2010) Spectroscopic evidence for intermediate species formed during aniline polymerization and polyaniline degradation. Phys Chem Chem Phys 12:10584–93. https://doi.org/10.1039/c002920c
Acknowledgements
We would like to thank the Veterinary Research and Development Center (Upper Northern Region), Department of Livestock Development, Thailand, and the Center of Excellence for Innovation in Chemistry, Chiang Mai University, Thailand, and Thammasat University, Thailand for partial support.
Funding
Research project supported by Thammasat University Research Fund, Contract No.TUFT 8/2565, Thammasat University, Thailand.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.L., and P.R.; methodology, J.L. and P.R.; validation, J.L.; formal analysis, J.L.; investigation, J.L.; resources, J.J. and P.R.; writing, original draft preparation, J.L.; writing, review and editing, J.J. and P.R.; visualization, J.L.; supervision, J.J. and P.R.; project administration, P.R.; funding acquisition, P.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This research did not involve human or animal samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lerdsri, J., Jakmunee, J. & Reanpang, P. Development of a sensitive electrochemical method to determine amitraz based on perylene tetracarboxylic acid/mesoporous carbon/Nafion@SPCEs. Microchim Acta 191, 228 (2024). https://doi.org/10.1007/s00604-024-06308-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06308-1