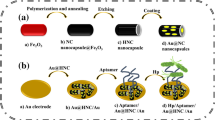
Abstract
In view of a large number of people infected with Helicobacter pylori (H. pylori) with great harm followed, there is an urgent need to develop a non-invasive, easy-to-operate, and rapid detection method, and to identify effective sterilization strategies. In this study, highly specific nanoprobes with nanozyme activity, Ag@Pt nanoparticles (NPs) with the antibody, were utilized as a novel lateral flow immunoassay (LFIA). The optical label (Ag@Pt NPs) was enhanced by the introduction of the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB) and compared with a gold nanoparticles (Au NPs) optical label. Under the optimal condition, Ag@Pt-LFIA and TMB-enhanced Ag@Pt-LFIA for H. pylori were successfully established, two of which were over twofold and 100-fold more sensitive than conventional visual Au NP-based LFIA, respectively. Furthermore, Ag@Pt NPs with the antibody irradiated with NIR laser (808 nm) at a power intensity of 550 mW/cm2 for 5 min exhibited a remarkable antibacterial effect. The nanoprobes could close to bacteria through effective interactions between antibodies and bacteria, thereby benefiting photothermal sterilization. Overall, Ag@Pt NPs provide promising applications in pathogen detection and therapeutic applications.
Graphical Abstract









Similar content being viewed by others
References
Rhee KH, Park JS, Cho MJ (2014) Helicobacter pylori: bacterial strategy for incipient stage and persistent colonization in human gastric niches. Yonsei Med J 55(6):1453–1466. https://doi.org/10.3349/ymj.2014.55.6.1453
Warren JR, Marshall B (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet (London, England) 1(8336):1273–1275
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet (London, England) 1(8390):1311–1315
Saxena A, Mukhopadhyay AK.Nandi SP (2020) Helicobacter pylori: perturbation and restoration of gut microbiome. J Biosci 45(1). https://doi.org/10.1007/s12038-020-00078-7
Dang BN, Graham DY (2017) Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol 14(7):383–384. https://doi.org/10.1038/nrgastro.2017.57
Graham DY, Dang BN, El-Serag HB (2019) Helicobacter pylori infection. N Engl J Med 381(6):587–588. https://doi.org/10.1056/NEJMc1905439
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118(12):3030–3044. https://doi.org/10.1002/ijc.21731
Mccoll KEL (2010) Helicobacter pylori infection. N Engl J Med 362(17):1597–1604. https://doi.org/10.1056/NEJMcp1001110
Konorev MR, Litviakov AM, Krylov IV (2000) Identification of Helicobacter pylori in stomach contents. Klin Lab Diagn 1:41–43
Chiba N, Van Zanten S (1999) C-13-Urea breath tests are the noninvasive method of choice for Helicobacter pylori detection. Can J Gastroenterol 13(8):681–683
Shuber AP, Ascano JJ, Boynton KA, Mitchell A, Frierson HF, El-Rifai W et al (2002) Accurate, noninvasive detection of Helicobacter pylori DNA from stool samples: potential usefulness for monitoring treatment. J Clin Microbiol 40(1):262–264. https://doi.org/10.1128/jcm.40.1.262-264.2002
Abrams DN, Koslowsky I, Matte G (2000) Pharmaceutical interference with the C-14 carbon urea breath test for the detection of Helicobacter pylori infection. J Pharm Pharm Sci 3(2):228–233
Gurbuz AK, Ozel AM, Narin Y, Yazgan Y, Baloglu H, Demirturk L (2005) Is the remarkable contradiction between histology and C-14 urea breath test in the detection of Helicobacter pylori due to false-negative histology or false-positive C-14 urea breath test? J Int Med Res 33(6):632–640. https://doi.org/10.1177/147323000503300604
Santolaria S, Lanas A, Benito R, Piazuelo E, Sainz R (2001) Serum CagA and VacA antibodies and risk for peptic ulcer disease in subjects with Helicobacter pylori infection. Med Clin 116(17):641–644. https://doi.org/10.1016/s0025-7753(01)71935-5
Gisbert JP, Pajares JM (2004) Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther 20(10):1001–1017. https://doi.org/10.1111/j.1365-2036.2004.02203.x
Chen L, Li X, Zou T, Wang T, Cui X, Chen Y et al (2019) Ultrasensitive detection of H. pylori in human feces based on immunomagnetic bead capture and fluorescent quantum dots. Analyst 144(13):4086–4092. https://doi.org/10.1039/c9an00193j
Wang T, Li X, Chen L, Zhang Y, Zheng Y, Yu L et al (2021) The preparation of bifunctional hybrid nano-flowers and their application in the enzyme-linked immunosorbent assay for Helicobacter pylori detection. Analyst 146(1):338–347. https://doi.org/10.1039/d0an01533d
Chotithammakul S, Cortie MB, Pissuwan D (2021) Comparison of single- and mixed-sized gold nanoparticles on lateral flow assay for albumin detection. Biosensors-Basel 11(7). https://doi.org/10.3390/bios11070209
Zhao M, Yao XL, Liu SJ, Zhang H, Wang LL, Yin XC et al (2021) Antibiotic and mammal IgG based lateral flow assay for simple and sensitive detection of Staphylococcus aureus. Food Chem 339. https://doi.org/10.1016/j.foodchem.2020.127955
Liu J, Yu QQ, Zhao GY, Dou WC (2020) Ultramarine blue nanoparticles as a label for immunochromatographic on-site determination of ractopamine. Microchim Acta 187(5). https://doi.org/10.1007/s00604-020-04270-2
Ben Aissa A, Araujo B, Julian E, Zanoni MVB, Pividori MI (2021) Immunomagnetic separation improves the detection of mycobacteria by paper-based lateral and vertical flow immunochromatographic assays. Sensors 21(18). https://doi.org/10.3390/s21185992
Wang CW, Yang XS, Zheng S, Cheng XD, Xiao R, Li QJ et al (2021) Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens Actuators B-Chem 345. https://doi.org/10.1016/j.snb.2021.130372
Cheng XD, Zheng S, Wang WQ, Han H, Yang XS, Shen WZ et al (2021) Synthesis of two-dimensional graphene oxide-fluorescent nanoprobe for ultrasensitive and multiplex immunochromatographic detection of respiratory bacteria. Chem Eng J 426. https://doi.org/10.1016/j.cej.2021.131836
He QY, Yang HY, Pan JK, Cui XP, Shen D, Eremin SA et al (2020) Lateral flow immunosensor for ferritin based on dual signal-amplified strategy by rhodium nanoparticles. Acs App Bio Mater 3(12):8849–8856. https://doi.org/10.1021/acsabm.0c01169
Pan JK, He QY, Lao ZT, Zou YK, Su JY, Li QL et al (2021) A bifunctional immunosensor based on osmium nano-hydrangeas as a catalytic chromogenic and tinctorial signal output for folic acid detection. Analyst 147(1):55–65. https://doi.org/10.1039/d1an01432c
Cui XP, He QY, Yang HY, Chen YS, Shen D, Eremin SA et al (2021) Development of enzyme-free single-step immunoassays for glycocholic acid based on palladium nanoparticle-mediated signal generation. Anal Bioanal Chem 413(23):5733–5742. https://doi.org/10.1007/s00216-021-03548-5
Yang HY, He QY, Chen YS, Shen D, Xiao HX, Eremin SA et al (2020) Platinum nanoflowers with peroxidase-like property in a dual immunoassay for dehydroepiandrosterone. Microchim Acta 187(11). https://doi.org/10.1007/s00604-020-04528-9
Cui ML, Zhou JD, Zhao Y, Song QJ (2017) Facile synthesis of iridium nanoparticles with superior peroxidase-like activity for colorimetric determination of H2O2 and xanthine. Sens Actuators B-Chem 243:203–210. https://doi.org/10.1016/j.snb.2016.11.145
Zambon CF, Basso D, Navaglia F, Mazza S, Razetti M, Fogar P et al (2004) Non-invasive diagnosis of Helicobacter pylori infection: simplified C-13-urea breath test, stool antigen testing, or DNA PCR in human feces in a clinical laboratory setting? Clin Biochem 37(4):261–267. https://doi.org/10.1016/j.clinbiochem.2003.12.004
Wu JC, Liu GL, Zhang ZH, Mou YL, Chen QA, Yang SL (1992) 15NH4+ excretion test: a new method for detection of Helicobacter pylori infection. J Clin Microbiol 30(1):181–184. https://doi.org/10.1128/jcm.30.1.181-184.1992
Monteiro L, Bonnemaison D, Vekris A, Petry KG, Bonnet J, Vidal R et al (1997) Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J Clin Microbiol 35(4):995–998. https://doi.org/10.1128/jcm.35.4.995-998.1997
Fischbach W, Malfertheiner P, Jansen PL, Bolten W, Bornschein J, Buderus S et al (2016) S2k-guideline Helicobacter pylori and gastroduodenal ulcer disease. Z Fur Gastroenterol 54(4):327–363. https://doi.org/10.1055/s-0042-102967
Roushani M, Sarabaegi M, Hosseini H, Pourahmad F (2022) Gold nanostructures integrated on hollow carbon N-doped nanocapsules as a novel high-performance aptasensing platform for Helicobacter pylori detection. J Mater Sci 57(1):589–597. https://doi.org/10.1007/s10853-021-06667-7
Fei Y, Fang R, Xiao LA, Zhang YQ, Fan K, Jiang YD et al (2022) The development of a colorimetric biosensing assay for the detection of Helicobacter pylori in feces. Anal Biochem 651. https://doi.org/10.1016/j.ab.2022.114737
Ibelli T, Templeton S, Levi-Polyachenko N (2018) Progress on utilizing hyperthermia for mitigating bacterial infections. Int J Hyperthermia 34(2): 144–156.https://doi.org/10.1080/02656736.2017.1369173
Funding
This work was financially supported by the Science and Technology Foundation Key R&D Program of Guangdong Province (2019B020209009; 2019B020218009), R&D Program of Guangdong Province Drug Administration (2021TDZ09; 2021YDZ06), Guangzhou Science and Technology Foundation (201903010034; 202102020682; 202201010308), GuangDong Basic and Applied Basic Research Foundation (2020A1515010952; 2021A1515220016; 2021A1515110331; 2022A1515011807), and the National Natural Science Foundation of China (No. 22108041).
Author information
Authors and Affiliations
Contributions
LZ: conceptualization, data curation, investigation, methodology, validation, writing—original draft; Li Ji: investigation, methodology; RL: conceptualization, investigation, methodology; HY: conceptualization, methodology; ML: conceptualization, methodology; JZ: supervision, writing—review and editing; SZ: funding acquisition, investigation, project administration, resources, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingjing Zhao contributed equally to this work and should be considered the co-corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Ji, L., Lin, M. et al. Hollow versatile Ag@Pt alloy nanoparticles with nanozyme activity for detection and photothermal sterilization of Helicobacter pylori. Microchim Acta 191, 330 (2024). https://doi.org/10.1007/s00604-024-06304-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06304-5