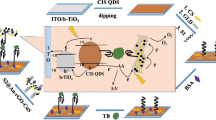
Abstract
A photoelectrochemical (PEC) sensor for the sensitive detection of thrombin (TB) was established. Co-sensitized combination of TiO2 nanoparticles combined with modified cadmium sulfide and cadmium telluride quantum dots (CdS/CdTe QDs) was utilized as a photoactive material. Successful growth of CdS/CdTe quantum dots on mesoporous TiO2 films occured by successive ion-layer adsorption and reaction. This interesting formation of co-sensitive structure is conducive to enhancing the photocurrent response by improving the use rate of light energy. Additionally, the step-level structure of CdS/CdTe QDs and TiO2 NPs shows a wide range of visible light absorption, facilitating the dissociation of excitons into free electrons and holes. Consequently, the photoelectric response of the PEC analysis platform is significantly enhanced. This constructed PEC aptasensor shows good detection of thrombin with a low detection limit (0.033 pM) and a wide linear range (0.0001–100 nM) in diluted actual human serum samples. In addition, this PEC aptasensor also has the characteristics of good stability and good reproducibility, which provides a novel insight for the quantitative measurement of other similar analytes.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data are available from the corresponding author upon reasonable request.
References
Zhang Y, Ranaei Pirmardan E, Barakat A, Hafezi-Moghadam A (2023) Breath biopsy reveals systemic immunothrombosis and its resolution through bioorthogonal dendritic nanoprobes. Adv Mater 35(45):2304903. https://doi.org/10.1002/adma.202304903
Wang Z, Zhao Y, Hou Y et al (2023) A thrombin-activated peptide-templated nanozyme for remedying ischemic stroke via thrombolytic and neuroprotective actions. Adv Mater 36(10):2210144. https://doi.org/10.1002/adma.202210144
Fang Y, Wang H-M, Gu Y-X et al (2020) Highly enhanced electrochemiluminescence luminophore generated by zeolitic imidazole framework-8-linked porphyrin and its application for thrombin detection. Anal. Chem. 92(4):3206–3212. https://doi.org/10.1021/acs.analchem.9b04938
Sun Y, Zhu X, Liu H et al (2020) Novel chemiluminescence sensor for thrombin detection based on dual-aptamer biorecognition and mesoporous silica encapsulated with iron porphyrin. ACS Appl. Mater . Interfaces 12(5):5569–5577. https://doi.org/10.1021/acsami.9b20255
Marar TT, Matzko CN, Wu J et al (2022) Thrombin spatial distribution determines protein C activation during hemostasis and thrombosis. Blood 139(12):1892–1902. https://doi.org/10.1182/blood.2021014338
Yang P, Guo X, Zhang J et al (2022) Picomolar thrombin detection by orchestration of triple signal amplification strategy with hierarchically porous Ti3C2Tx MXene electrode material-catalytic hairpin assembly reaction-metallic nanoprobes. Biosens Bioelectron 208:114228. https://doi.org/10.1016/j.bios.2022.114228
Zhang Q, Liu Q, Liu Y et al (2023) PEC thrombin aptasensor based on Ag-Ag2S decorated hematite photoanode with signal-down effect of precipitation catalyzed by G-quadruplexes/hemin. Biosens Bioelectron 232:115321. https://doi.org/10.1016/j.bios.2023.115321
Girish A, Jolly K, Alsaadi N et al (2022) Platelet-inspired intravenous nanomedicine for injury-targeted direct delivery of thrombin to augment hemostasis in coagulopathies. ACS Nano 16(10):16292–16313. https://doi.org/10.1021/acsnano.2c05306
Deng X, Wu S, Li Z, Zhao Y, Duan C (2020) Ratiometric detection of DNA and protein in serum by a universal tripyridinyl Ru(II) complexencapsulated SiO2@polydopamine fluorescence nanoplatform. Anal Chem 92(24):15908–15915. https://doi.org/10.1021/acs.analchem.0c03306
Dong J, Willner I (2023) Transient transcription machineries modulate dynamic functions of g-quadruplexes: temporal regulation of biocatalytic circuits, gene replication and transcription. Angew Chem Int Ed Engl 62(33): e202307898. https://doi.org/10.1002/anie.202307898
Riccardi C, Napolitano E, Platella C, Musumeci D, Montesarchio D (2021) G-quadruplex based aptamers targeting human thrombin: discovery, chemical modifications and antithrombotic effects. Pharmacol Ther 217:107649. https://doi.org/10.1016/j.pharmthera.2020.107649
Fan X, Ouyang X, Zhou Z et al (2023) A highly selective self-powered sensor based on the upconversion nanoparticles/CdS nanospheres for chlorpyrifos detection. Biosens Bioelectron 237:115475. https://doi.org/10.1016/j.bios.2023.115475
Deng Y, He R, Lu H et al (2023) Visible-light driven and efficient photoelectrochemical aptasensor constructed with N-doped carbon quantum dots-decorated TiO2 nanorods for determination of di-2-ethylhexyl phthalate. Chem Eng J 468:143583. https://doi.org/10.1016/j.cej.2023.143583
Li H, Sheng W, Haruna S A et al (2023) Recent progress in photoelectrochemical sensors to quantify pesticides in foods: theory, photoactive substrate selection, recognition elements and applications. Trends Analyt Chem 164:117108. https://doi.org/10.1016/j.trac.2023.117108
Zhu J-H, Mei L-P, Wang A-J, Song Y-Y, Feng J-J (2023) Integration of phosphate functionalized Pt/TiO2 and Ru(bpy)32+ sensitization for ultrasensitive assay of adenosine deaminase activity on a novel split-typed PEC aptasensor. Biosens Bioelectron 226:115141. https://doi.org/10.1016/j.bios.2023.115141
Liang X, Zhao J, Ma Z (2020) Improved binding induced self-assembled DNA to achieve ultrasensitive electrochemical proximity assay. Sens Actuators B Chem 304:127278. https://doi.org/10.1016/j.snb.2019.127278
Liu B, Ge Y, Lu Y et al (2023) An NIR light-responsive “on-off-on” photoelectrochemical aptasensor for carcinoembryonic antigen assay based on Y-shaped DNA. Biosens Bioelectron 229:115241. https://doi.org/10.1016/j.bios.2023.115241
Hu R, Ren X X, Song P et al (2023) Hollow cage-like PtCu nanozyme-regulated photo-activity of porous CdIn2S4/SnO2 heterojunctions for ultrasensitive PEC sensing of streptomycin. Biosens Bioelectron 236:115425. https://doi.org/10.1016/j.bios.2023.115425
Ghosh Dastidar M, Schumann U, Lu T et al (2023) A simple yet highly sensitive and selective aptasensor architecture for rapid and portable miRNA detection. Chem Eng J 454:140186. https://doi.org/10.1016/j.cej.2022.140186
Alkhamis O, Canoura J, Willis C et al (2023) Comparison of aptamer signaling mechanisms reveals disparities in sensor response and strategies to eliminate false signals. J Am Chem Soc 145(22):12407–12422. https://doi.org/10.1021/jacs.3c03640
Zheng L, Guo Q, Yang C et al (2023) Electrochemiluminescence and photoelectrochemistry dual-signal immunosensor based on Ru(bpy)32+ functionalized MOF for prostate-specific antigen sensitive detection. Sens Actuators B Chem 379:133269. https://doi.org/10.1016/j.snb.2022.133269
Wang K, Yang J, Yang X, Guo Q, Nie G (2022) Photoelectrochemical nanoprobe for combined monitoring of Cu2+and β-amyloid peptide. Microchem J 182:107952. https://doi.org/10.1016/j.microc.2022.107952
Guo Y, Dai X, Zhang Y et al (2023) Universal hydrogen-treated TiO2 nanorod array/Ti2COX MXene PEC aptamer sensor modulated by the transport characteristic of photogenerated holes. Anal Chem 95(19):7560–7568. https://doi.org/10.1021/acs.analchem.3c00046
Zhang Y, Wu G, Chen Y et al (2023) Enhancing charge separation/transfer based on Co(II) doped tubular g-C3N4 for photoelectrochemical atrazine aptasensing. J Environ Chem Eng 11(3):110173. https://doi.org/10.1016/j.jece.2023.110173
Abdelkarim O, Mirzaei A, Selopal G S et al (2022) Constructing quantum dots sensitized TiO2 nanotube P-N heterojunction for photoelectrochemical hydrogen generation. Chem Eng J 446:137312. https://doi.org/10.1016/j.cej.2022.137312
Liu J, Luo Z, Mao X et al (2022) Recent advances in self-supported semiconductor heterojunction nanoarrays as efficient photoanodes for photoelectrochemical water splitting. Small 18(48):e2204553. https://doi.org/10.1002/smll.202204553
Boochakiat S, Inceesungvorn B, Nattestad A, Chen J (2023) Bismuth‐based oxide photocatalysts for selective oxidation transformations of organic compounds. Chem Nano Mat 9(7):e202300140. https://doi.org/10.1002/cnma.202300140
Tantraviwat D, Nattestad A, Chen J, Inceesungvorn B (2023) Enhanced photoactivity and selectivity over BiOI-decorated Bi2WO6 microflower for selective oxidation of benzylamine: Role of BiOI and mechanism. J Colloid Interf Sci 629(Pt A):854–863. https://doi.org/10.1016/j.jcis.2022.09.020
Hu Z, Zhou M, Maitlo H A et al (2023) Novel dual-photoelectrode photoelectrocatalytic system based on TiO2 nanoneedle arrays photoanode and nitrogen-doped carbon dots/Co3O4 photocathode for efficient water purification at low/no applied voltage. Appl Catal B 331:122676. https://doi.org/10.1016/j.apcatb
Wu J, Tao Y, Zhang C et al (2023) Activation of chloride by oxygen vacancies-enriched TiO2 photoanode for efficient photoelectrochemical treatment of persistent organic pollutants and simultaneous H2 generation. J Hazard Mater 443(Pt B):130363. https://doi.org/10.1016/j.jhazmat.2022.130363
.Amani Ghadim A R, Mousavi M, Bayat F (2022) Dysprosium doping in CdTe@CdS type II core/shell and cosensitizing with CdSe for photocurrent and efficiency enhancement in quantum dot sensitized solar cells. J Power Sources 539:231624. https://doi.org/10.1016/j.jpowsour.2022.231624
Jian J-X, Xie L-H, Mumtaz A et al (2023) Interface-engineered Ni-coated CdTe heterojunction photocathode for enhanced photoelectrochemical hydrogen evolution. ACS Appl Mater Interfaces 15(17):21057–21065. https://doi.org/10.1021/acsami.3c01476
Cai J, Sheng P, Zhou L et al (2013) Label-free photoelectrochemical immunosensor based on CdTe/CdS co-sensitized TiO2 nanotube array structure for octachlorostyrene detection. Biosens Bioelectron 50:66–71. https://doi.org/10.1016/j.bios.2013.05.040
Chen X, Shen X, Shen S, Reese MO, Hu S (2020) Stable CdTe photoanodes with energetics matching those of a coating intermediate band. ACS Energy Lett. 5(6):1865–1871. https://doi.org/10.1021/acsenergylett.0c00603
Fan G-C, Han L, Zhu H, Zhang J-R, Zhu J-J (2014) Ultrasensitive photoelectrochemical immunoassay for matrix metalloproteinase-2 detection based on CdS:Mn/CdTe cosensitized TiO2 nanotubes and signal amplification of SiO2@Ab2 conjugates. Anal. Chem. 86(24):12398–12405. https://doi.org/10.1021/ac504027d
Du D, Wang L, Ding D et al (2023) One-step synthesis of aqueous CdTe/CdSe composite QDs toward efficiency enhancement of solar cell. Chem Eng J 461:142040. https://doi.org/10.1016/j.cej.2023.142040
Wang J, Wang L, Su X, Chen R (2022) Bandgap engineering of CdTe/CdSe rod-shaped core/shell and CdTeSe ellipsoidal alloy quantum dots with tunable and intense emission. J Alloys Compd 920:165907. https://doi.org/10.1016/j.jallcom.2022.165907
Ghazzal M N, Wojcieszak R, Raj G, Gaigneaux EM (2014) Study of mesoporous CdS-quantum-dot-sensitized TiO2 films by using X-ray photoelectron spectroscopy and AFM. Beilstein J Nanotechnol 5:68–76. https://doi.org/10.3762/bjnano.5.6
Cao H, Liu F, Tai Y et al (2023) Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem Eng J 466:143219. https://doi.org/10.1016/j.cej.2023.143219
Lu C, Drichel A, Chen J et al (2021) Sensibilization of P-NiO with ZnSe/CdS and CdS/ZnSe quantum dots for photoelectrochemical water reduction. Nanoscale 13(2):869–877. https://doi.org/10.1039/d0nr06993k
Zeng G, Zhang J, Li B et al (2015) Effect of deposition temperature on the properties of CdTe thin films prepared by close-spaced sublimation. J Electron Mater 44(8):2786–2791. https://doi.org/10.1007/s11664-015-3739-z
AbuEl-Rub KM, Hahn SR, Tari S, Dissanayake MAKL (2012) Effects of CdCl2 heat treatment on the morphological and chemical properties of CdTe/CdS thin films solar cells. Appl Surf Sci 258(16):6142–6147. https://doi.org/10.1016/j.apsusc.2012.03.020
Yue D, Qian X, Kan M et al (2017) Sulfurated [NiFe]-based layered double hydroxides nanoparticles as efficient co-catalysts for photocatalytic hydrogen evolution using CdTe/CdS quantum dots. Appl Catal B 209:155–160. https://doi.org/10.1016/j.apcatb.2017.02.075
Molaei M, Hasheminejad H, Karimipour M (2015) Synthesizing and investigating photoluminescence properties of CdTe and CdTe@CdS core-shell quantum dots (QDs): a new and simple microwave activated approach for growth of CdS shell around CdTe core. Electron Mater Lett 11(1):7–12. https://doi.org/10.1007/s13391-014-4039-0
Zhu C, Zhu W, Xu L, Zhou X (2019) A label-free electrochemical aptasensor based on magnetic biocomposites with Pb2+-dependent DNAzyme for the detection of thrombin. Anal Chim Acta 104:721–27. https://doi.org/10.1016/j.aca.2018.09.040
Zhang Q, Liu X, Wang H et al (2021) Photoelectrochemical thrombin biosensor based on perylene-3,4,9,10-tetracarboxylic acid and Au co-functionalized ZnO nanorods with signal-off quenching effect of Ag@Ag2S, Analyst 146(3):855–863. https://doi.org/10.1039/d0an02167a
Yang C, Li Z, Tian Y, Guo Q, Nie G (2020) A simple label-free photoelectrochemical aptasensor for ultrasensitive detection of thrombin. Microchem J 159:105452. https://doi.org/10.1016/j.microc.2020.105452
Paimard G, Gholivand M B, Shamsipur M, Ahmadi E, Shahlaei M (2021) Introduction of a thrombin sensor based on its interaction with dabigatran as an oral direct thrombin inhibitor. Mater Sci Eng C 119:111417. https://doi.org/10.1016/j.msec.
Wang X, Gao F, Gong Y et al (2019) Electrochemical aptasensor based on conductive supramolecular polymer hydrogels for thrombin detection with high selectivity. Talanta 205:120140. https://doi.org/10.1016/j.talanta.2019.120140
Wu H, Xi K, Xiao S et al (2020) Self-assembled perylenetetracarboxylic acid-reduced graphene oxide film for high-sensitive impedimetric determination of thrombin. Surf Coat Technol 402:126491. https://doi.org/10.1016/j.surfcoat.2020.126491
Cui H, Wu W, Xu H et al (2020) A homogeneous strategy of target-triggered catalytic hairpin assembly for thrombin signal amplification. Microchem J 159:105537. https://doi.org/10.1016/j.microc.2020.105537
Li J, Zhou W, Yuan R, Xiang Y (2018) Aptamer proximity recognition-dependent strand translocation for enzyme-free and amplified fluorescent detection of thrombin via catalytic hairpin assembly. Anal Chim Acta 103(8):126–131. https://doi.org/10.1016/j.aca.2018.07.011
Liu Y, Zhu Z, Wang C et al (2019) Responsive surface bioaffinity binding to construct flexible and sensitive electrochemical aptasensors. Analyst 144(6):2130–2137. https://doi.org/10.1039/c8an02313a
Yang X, Lv J, Yang Z, Yuan R, Chai Y (2017) A sensitive electrochemical aptasensor for thrombin detection based on electroactive Co-based metalorganic frameworks with target-triggering NESA strategy. Anal Chem 89(21):11636–11640. https://doi.org/10.1021/acs.analchem.7b03056
Grieshaber D, MacKenzie R, Vӧrӧs J, Reimhult E (2008) Electrochemical biosensors-sensor principles and architectures. Sensors 8(3):1400–1458. https://doi.org/10.3390/s80314000
Ghanbary E, Asiabani Z, Hosseini N et al (2020) The development of a new modified graphite pencil electrode for quantitative detection of Gibberellic acid (GA3) herbal hormone. Microchem J 157:105005. https://doi.org/10.1016/j.microc
Rostami-Javanroudi S, Babakhanian A (2021) New electrochemical sensor for direct quantification of vitamin K in human blood serum. Microchem J 163:105716. https://doi.org/10.1016/j.microc.2020.105716
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51973102); Natural Science Foundation of Shandong Province (ZR2022MB042; ZR2019MB067); Qinghai Provincial Basic Research Program (2021-ZJ-710); Innovation Ability Improvement Project of Science and Technology Small and Medium-Size Enterprise in Shandong Province (2022TSGC1121); Talent Fund of QUST (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics declarations
This research did not involve human or animal samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Lu, Y., Liu, D. et al. Highly efficient photoelectrochemical aptasensor based on CdS/CdTe QDs co-sensitized TiO2 nanoparticles designed for thrombin detection. Microchim Acta 191, 216 (2024). https://doi.org/10.1007/s00604-024-06279-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06279-3